Home /
Expert Answers /
Algebra /
milk-of-magnesia-is-a-suspension-of-magnesium-hydroxide-in-water-although-mathrm-mg-mathrm-o-pa123
(Solved): Milk of magnesia is a suspension of magnesium hydroxide in water. Although \( \mathrm{Mg}(\mathrm{O ...
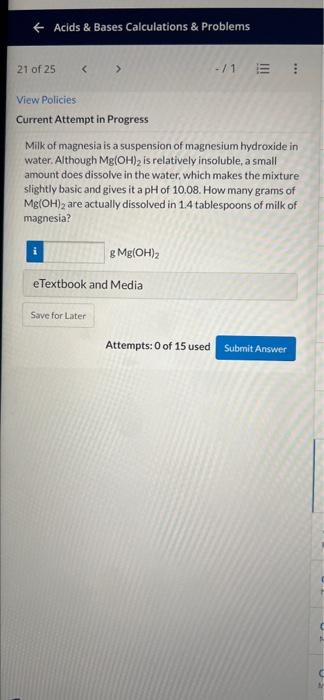
Milk of magnesia is a suspension of magnesium hydroxide in water. Although \( \mathrm{Mg}(\mathrm{OH})_{2} \) is relatively insoluble, a small amount does dissolve in the water, which makes the mixture slightly basic and gives it a \( \mathrm{pH} \) of \( 10.08 \). How many grams of \( \mathrm{Mg}(\mathrm{OH})_{2} \) are actually dissolved in \( 1.4 \) tablespoons of milk of magnesia? \[ \mathrm{g} \mathrm{Mg}(\mathrm{OH})_{2} \]
Expert Answer
Given that, the pH of the milk of magnesia is 10.08. The objective is to find the amount of Mg(OH)2 that can be dissolved in 1.4 tablespoons of milk o