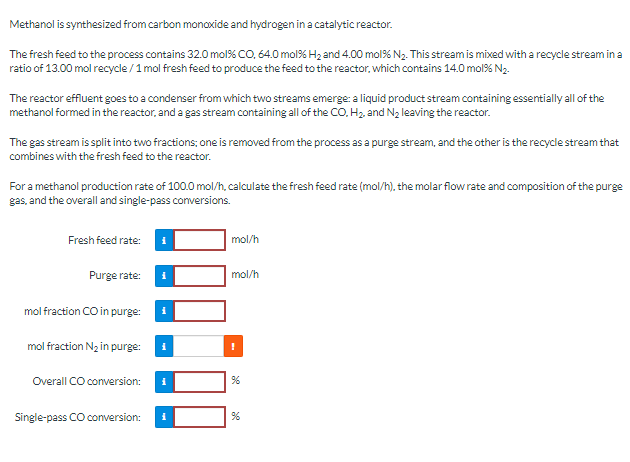
(Solved): Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed to ...
Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed to the process contains
32.0mol%CO,64.0mol%H_(2)and
4.00mol%N_(2). This stream is mixed with a recycle stream in a ratio of 13.00 mol recycle
()/(1)mol fresh feed to produce the feed to the reactor, which contains
14.0mol%N_(2). The reactor effluent goes to a condenser from which two streams emerge: a liquid product stream containing essentially all of the methanol formed in the reactor, and a gas stream containing all of the
CO,H_(2), and
N_(2)leaving the reactor. The gas stream is split into two fractions; one is removed from the process as a purge stream, and the other is the recycle stream that combines with the fresh feed to the reactor. For a methanol production rate of
100.0mo(l)/(h), calculate the fresh feed rate
(mo(l)/(h)), the molar flow rate and composition of the purge gas, and the overall and single-pass comversions.