Home /
Expert Answers /
Chemistry /
methanol-can-be-formed-by-the-following-unbalanced-equation-below-what-is-the-mass-in-grams-of-meth-pa718
(Solved): Methanol can be formed by the following UNBALANCED equation below. What is the mass in grams of meth ...
Methanol can be formed by the following UNBALANCED equation below. What is the mass in grams of methanol that can be formed from 137 molecules of CO? H?(g) + CO(g) ? CH3OH(I).
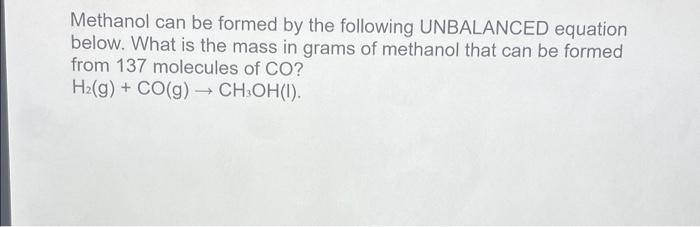
Methanol can be formed by the following UNBALANCED equation below. What is the mass in grams of methanol that can be formed from 137 molecules of CO?