Home /
Expert Answers /
Chemical Engineering /
methane-is-burned-in-a-furnace-using-10-excess-air-due-to-poor-irnace-design-some-incom-pa626
(Solved): Methane is burned in a furnace using \( 10 \% \) excess air. Due to poor irnace design, some incom ...
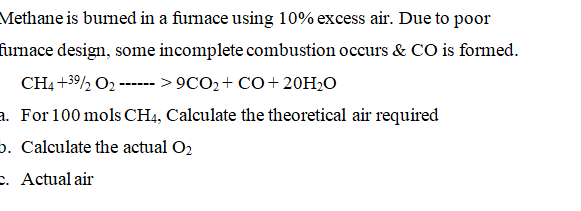
Methane is burned in a furnace using \( 10 \% \) excess air. Due to poor irnace design, some incomplete combustion occurs \& \( \mathrm{CO} \) is formed. \( \mathrm{CH}_{4}+39 / 2 \mathrm{O}_{2} \cdots>9 \mathrm{CO}_{2}+\mathrm{CO}+20 \mathrm{H}_{2} \mathrm{O} \) For \( 100 \mathrm{mols}^{-\mathrm{CH}_{4}} \), Calculate the theoretical air required Calculate the actual \( \mathrm{O}_{2} \) Actual air
Expert Answer
Given entities, Methane is burned with 10%excess air, We know that, Excess air = {(mole of air feed) - (mole