Home /
Expert Answers /
Chemistry /
match-each-compound-with-its-major-type-of-intermolecular-force-hco-c3h8-k2co3-h20-xe-atoms-dip-pa651
Expert Answer
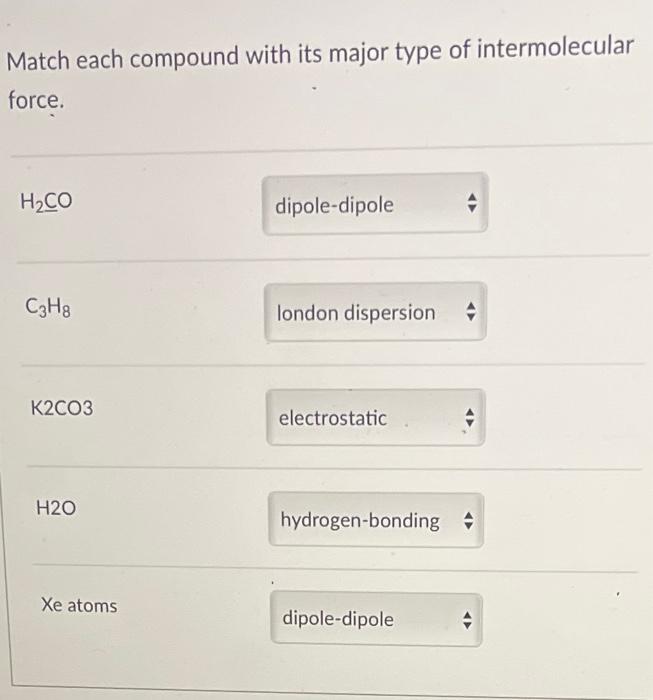
(1). H2CO H2CO is a polar molecule and will have both dipole-dipole forces and London dispersion forces. (2). C3H8 London forces are the only intermolecular force that propane (C3H8) molecules experience. (3). K2CO3 K2CO3 is an ionic compound that ha