Home /
Expert Answers /
Chemistry /
magnesium-metal-is-reacted-with-hydrochloric-acid-to-produce-hydrogen-gas-a-sample-of-hydrogen-gas-pa740
(Solved): Magnesium metal is reacted with hydrochloric acid to produce hydrogen gas. A sample of hydrogen gas ...
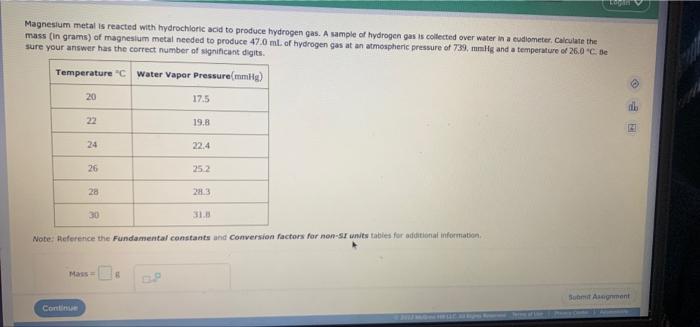
Magnesium metal is reacted with hydrochloric acid to produce hydrogen gas. A sample of hydrogen gas is collected over water in a cudiometer. Calculate the mass (in grams) of magnesium metal needed to produce 47.0 ml. of hydrogen gas at an atmospheric pressure of 739, mmHg and a temperature of 26.0 "C. Be sure your answer has the correct number of significant digits. Temperature "C Water Vapor Pressure(mmHg) 20 22 Continue 24 26 28 Mass= 6 17.5 19.8 22.4 25.2 28.3 Note: Reference the Fundamental constants and Conversion factors for non-SI units tables for additional information. 31.8 Serew of HPO Submit Asognment