Home /
Expert Answers /
Chemistry /
lithium-and-chlorine-react-according-to-the-balanced-chemical-equation-shown-below-if-the-reaction-pa342
(Solved): Lithium and chlorine react according to the balanced chemical equation shown below. If the reaction ...
Lithium and chlorine react according to the balanced chemical equation shown below. If the reaction is performed at STP, how many liters of chlorine are required to completely react with 12.3 grams of lithium?
2 Li(s) + Cl2(g)-> 2 LiCI(s)
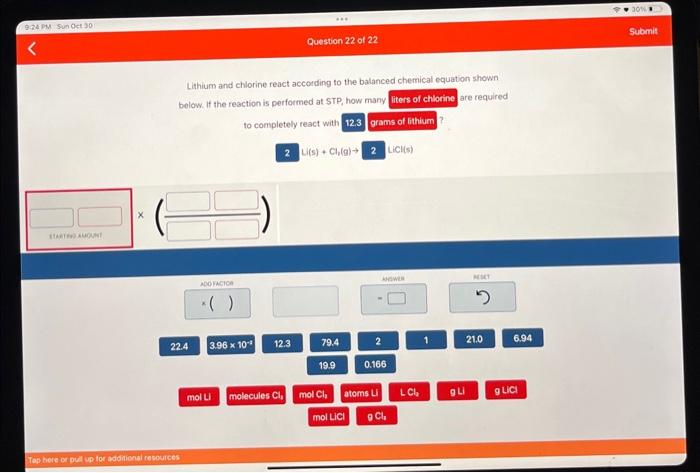

Lithium and chlorine react according to the balanced chemical equation shown are required below. If the reaction is perfotmed at STP, how many to completely react with \[ \mathrm{Li}(\mathrm{s})+\mathrm{Cl}_{2}(\mathrm{~g}) \rightarrow \mathrm{LiCl}(\mathrm{s}) \]