Home /
Expert Answers /
Chemistry /
lead-iv-oxide-dissolves-in-concentrated-hydrochloric-acid-according-to-the-pa667
(Solved): Lead (IV) oxide dissolves in concentrated hydrochloric acid according to the ...
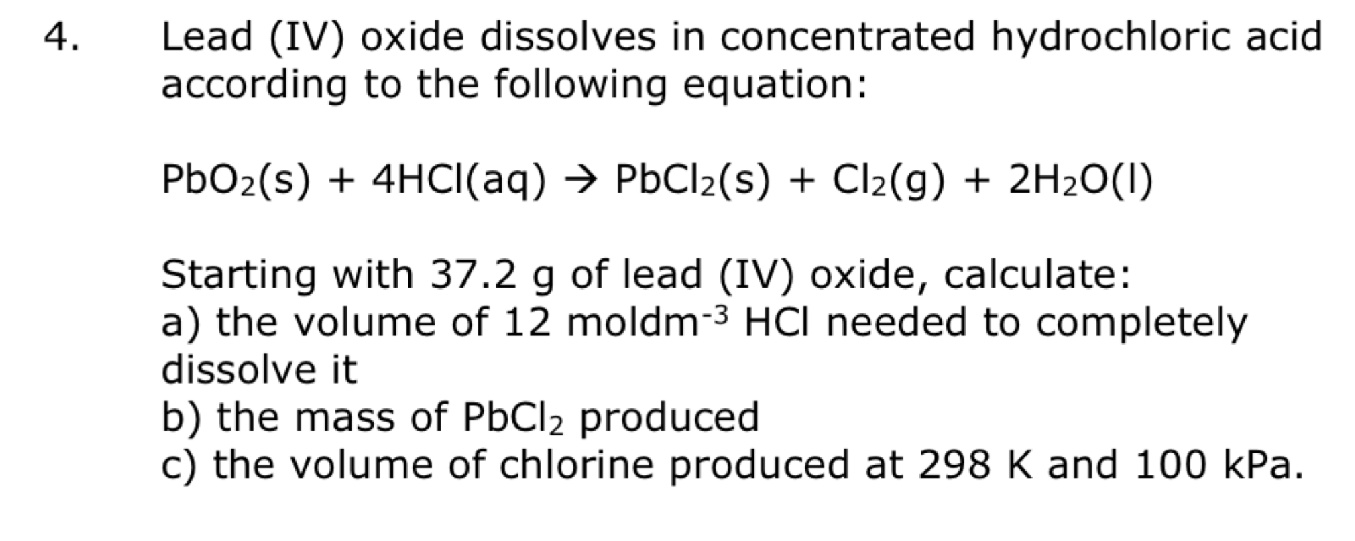
Lead (IV) oxide dissolves in concentrated hydrochloric acid according to the following equation: Starting with of lead (IV) oxide, calculate: a) the volume of 12 moldm needed to completely dissolve it b) the mass of produced c) the volume of chlorine produced at and .
Expert Answer
PbO2+4HCl = PbCl2+Cl2+2H2O ?Mol of PbO2=mass/molar mass =37.2g ÷ 239 2g/mol=0.156mol1)?Mol of HCl reacted =mol of