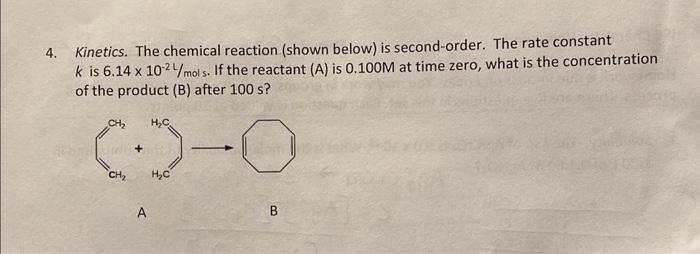Home /
Expert Answers /
Chemistry /
kinetics-the-chemical-reaction-shown-below-is-second-order-the-rate-constant-k-is-6-14102-pa258
Expert Answer
The rate law for a second order reaction A?Bis,Rate=k[A]2?d[A]dt=k[A]2At time t=0, we have [A0] and at time t = t, we have [A].Integrating the above d