Home /
Expert Answers /
Chemistry /
isopentyl-alcohol-88-15g-mol-acetic-acid-60-05g-mol-isopentyl-acetate-130-18g-mol-water-18-02-pa253
(Solved): isopentyl alcohol 88.15g/mol acetic acid 60.05g/mol isopentyl acetate 130.18g/mol water 18.02 ...
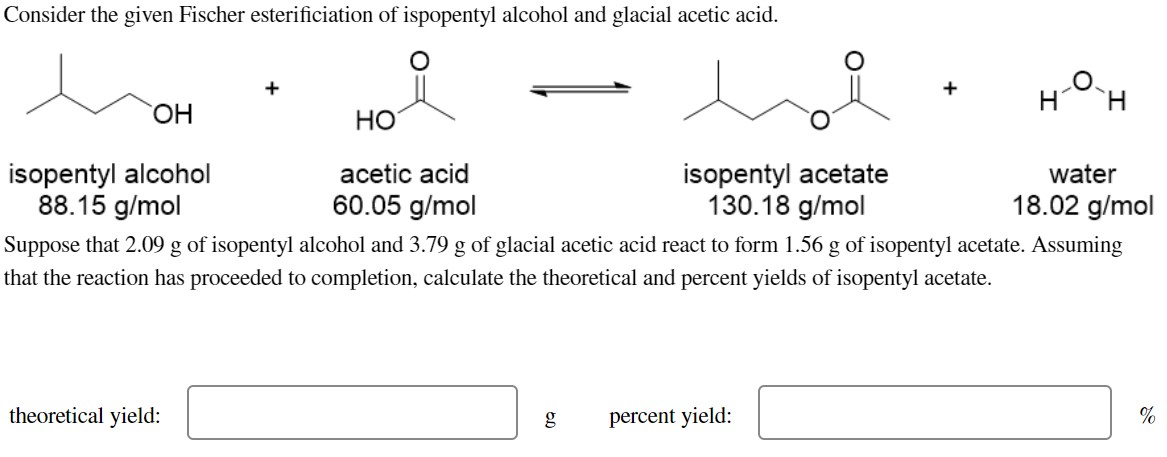
isopentyl alcohol acetic acid isopentyl acetate water Suppose that of isopentyl alcohol and of glacial acetic acid react to form of isopentyl acetate. Assuming that the reaction has proceeded to completion, calculate the theoretical and percent yields of isopentyl acetate. theoretical yield: percent yield:
Expert Answer
The balanced chemical equation for Fischer esterification of isopentyl alcohol (C5H12O) and glacial ...