Home /
Expert Answers /
Chemistry /
into-a-separatory-funnel-t-amyl-alcohol-5-07-g-has-conc-mathrm-hcl-17-45-mathrm-ml-pa955
(Solved): Into a separatory funnel, t-amyl alcohol (5.07 g) has conc. \( \mathrm{HCl}(17.45 \mathrm{~mL}) \) ...
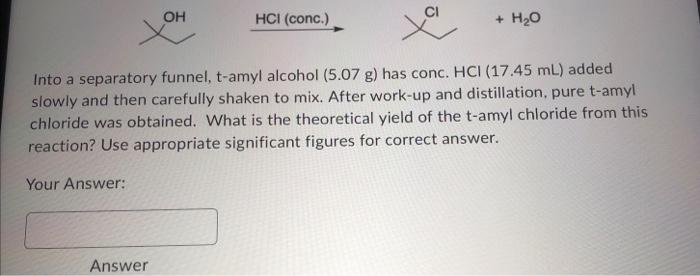

Into a separatory funnel, t-amyl alcohol (5.07 g) has conc. \( \mathrm{HCl}(17.45 \mathrm{~mL}) \) added slowly and then carefully shaken to mix. After work-up and distillation, pure t-amyl chloride was obtained. What is the theoretical yield of the \( t \)-amyl chloride from this reaction? Use appropriate significant figures for correct answer. Your Answer: Answer
The synthesis of \( \mathrm{t} \)-Amyl chloride follows an SN1 pathway. What is the nucleophile in this reaction? t-amyl alcohol \( \mathrm{OH}^{-} \) \( \mathrm{H}^{+} \) \( \mathrm{Cl}^{-} \) \( \mathrm{HCl} \)