Home /
Expert Answers /
Chemistry /
interparticle-forces-determine-the-dominant-interparticle-force-present-in-these-substances-ma-pa841
(Solved): Interparticle forces Determine the dominant interparticle force present in these substances. \[ \ma ...
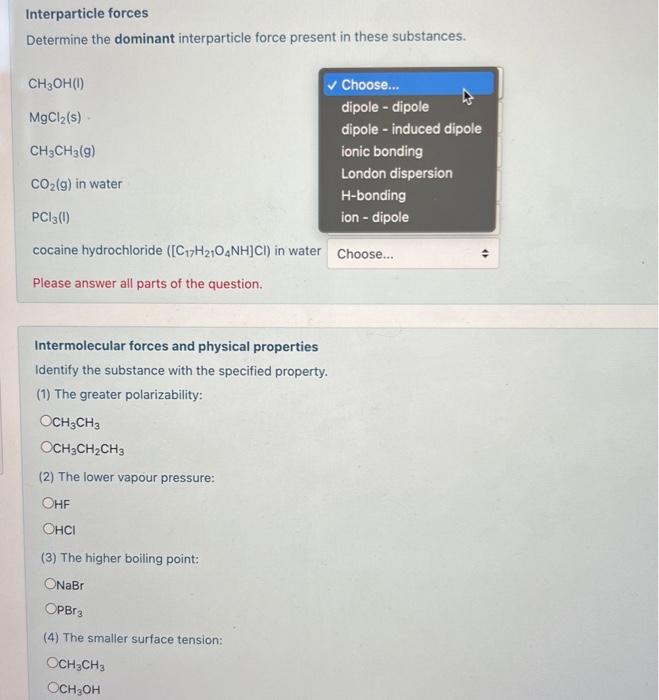
Interparticle forces Determine the dominant interparticle force present in these substances. \[ \mathrm{CH}_{3} \mathrm{OH}(\mathrm{I}) \] \( \mathrm{MgCl}_{2}(\mathrm{~s}) \) \( \mathrm{CH}_{3} \mathrm{CH}_{3}(\mathrm{~g}) \) \( \mathrm{CO}_{2}(\mathrm{~g}) \) in water \( \mathrm{PCl}_{3}(\mathrm{l}) \) cocaine hydrochloride \( \left(\left[\mathrm{C}_{17} \mathrm{H}_{21} \mathrm{O}_{4} \mathrm{NH}\right] \mathrm{Cl}\right) \) in water Please answer all parts of the question. Intermolecular forces and physical properties Identify the substance with the specified property. (1) The greater polarizability: \[ \begin{array}{l} \mathrm{CH}_{3} \mathrm{CH}_{3} \\ \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{3} \end{array} \] (2) The lower vapour pressure: \( \mathrm{HF} \) (3) The higher boiling point: \( \mathrm{NaBr} \) \[ \mathrm{PBr}_{3} \] (4) The smaller surface tension: \[ \begin{array}{l} \mathrm{CH}_{3} \mathrm{CH}_{3} \\ \mathrm{CH}_{3} \mathrm{OH} \end{array} \]