Home /
Expert Answers /
Chemistry /
in-the-following-lewis-structure-for-perchlorate-chlorine-has-a-formal-charge-of-and-an-oxidation-pa832
Expert Answer
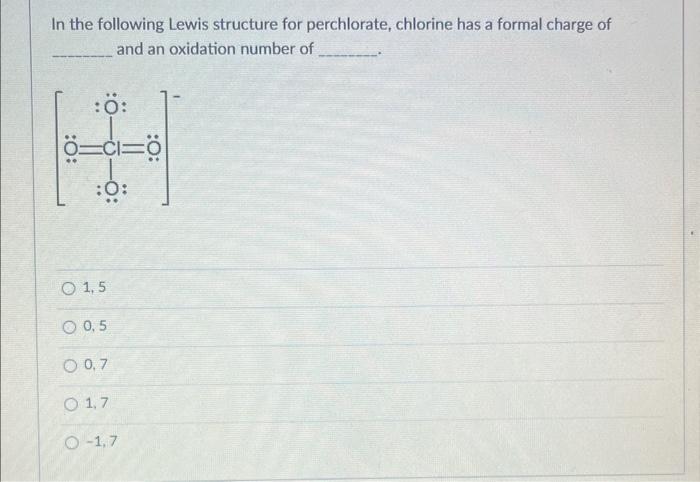
The structure of perchlorate is given. We need to find the formal charge of chlorine and an oxidat