Home /
Expert Answers /
Chemistry /
in-the-fist-step-of-this-reaction-urea-is-broken-down-into-isocyanic-acid-and-ammonia-molamine-pa402
(Solved): In the fist step of this reaction, urea is broken down into isocyanic acid and ammonia: Molamine, \ ...
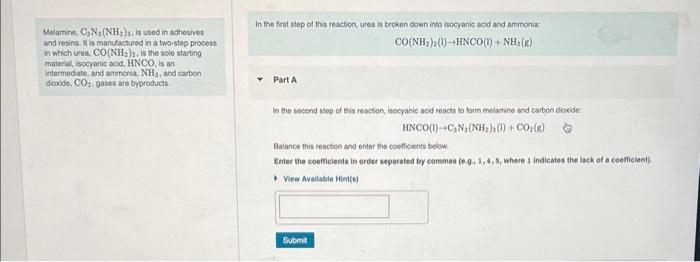
In the fist step of this reaction, urea is broken down into isocyanic acid and ammonia: Molamine, \( \mathrm{O}_{3} \mathrm{~N}_{3}\left(\mathrm{NH}_{2}\right)_{3} \), is used in adhestves: \[ \mathrm{CO}\left[\mathrm{NH}_{2}\right)_{2}(\mathrm{l}) \rightarrow \mathrm{HNCO}(\mathrm{l})+\mathrm{NH}_{1}(\mathrm{~g}) \] and resint. It is manufactured in a two-step process in which urea, \( \mathrm{CO}\left(\mathrm{NH}_{2} / 2\right. \), is the sole staring material, socyanic acid, HNCO, is an intermediate, and ammona. \( \mathrm{NH}_{3} \), and carbon doude, \( \mathrm{CO}_{2} \), gases are byproducts Part A In the second step of this resction, isocyanic acd reacts to form melstine and caiben dioxide: \[ \mathrm{HNCO}(1)+\mathrm{C}_{2} \mathrm{~N}_{3}\left(\mathrm{NH}_{2}\right)_{1}(1)+\mathrm{CO}_{2}(8) \] Balance this reactign and onter the coellicients below. Enter the coefticients in order separated by commas \( (0.9,1,4,5 \), where s indicates the lack of a coetficient)
Expert Answer
Solution: Given chemical equation to be balnced, HNCO (l) C3N3(NH2)3 (l) + CO2 (g) Steps for balancing a chemical equation: Step 1: Count each atoms in both reactant side and product side Reactant side: One hydrogen One Nitrogen One carbon One oxygen