Home /
Expert Answers /
Physics /
in-aluminum-calorimeter-with-a-mass-of-100g-contains-250g-of-water-the-calorimeter-and-water-a-pa860
(Solved): In aluminum calorimeter with a mass of 100g contains 250g of water. The calorimeter and water a ...
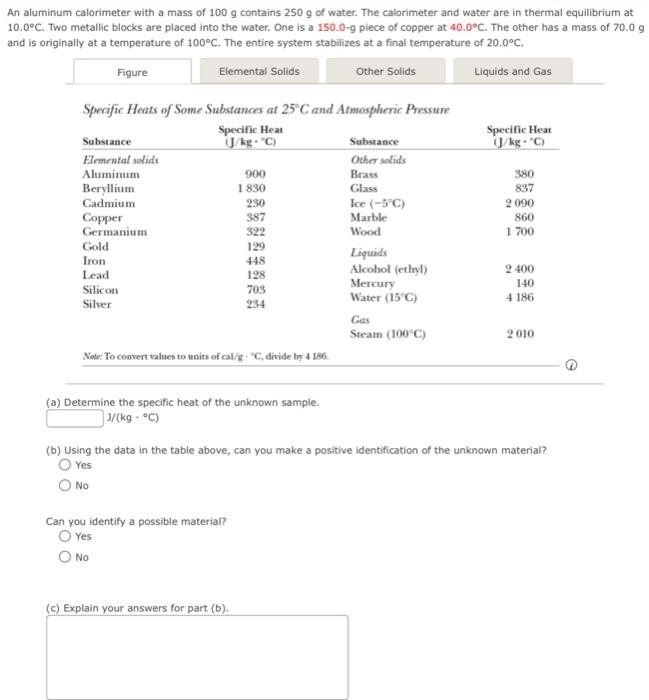
In aluminum calorimeter with a mass of contains of water. The calorimeter and water are in thermal equilibrium at . Two metallic blocks are placed into the water. One is a piece of copper at . The other has a mass of 70.0 ind is originally at a temperature of . The entire system stabilizes at a final temperature of . Specific Heats of Some Substances at and Atmospheric Pressure Note To convert values to units of cal/g - "C, divide by 4.186. (1) (a) Determine the specific heat of the unknown sample. (b) Using the data in the table above, can you make a positive identification of the unknown material? Yes No Can you identify a possible material? Yes No