Home /
Expert Answers /
Chemical Engineering /
in-a-fuel-cell-experiment-methanol-left-mathrm-ch-3-mathrm-oh-right-is-oxidised-to-pa244
(Solved): In a fuel cell experiment, methanol \( \left(\mathrm{CH}_{3} \mathrm{OH}\right) \) is oxidised to ...
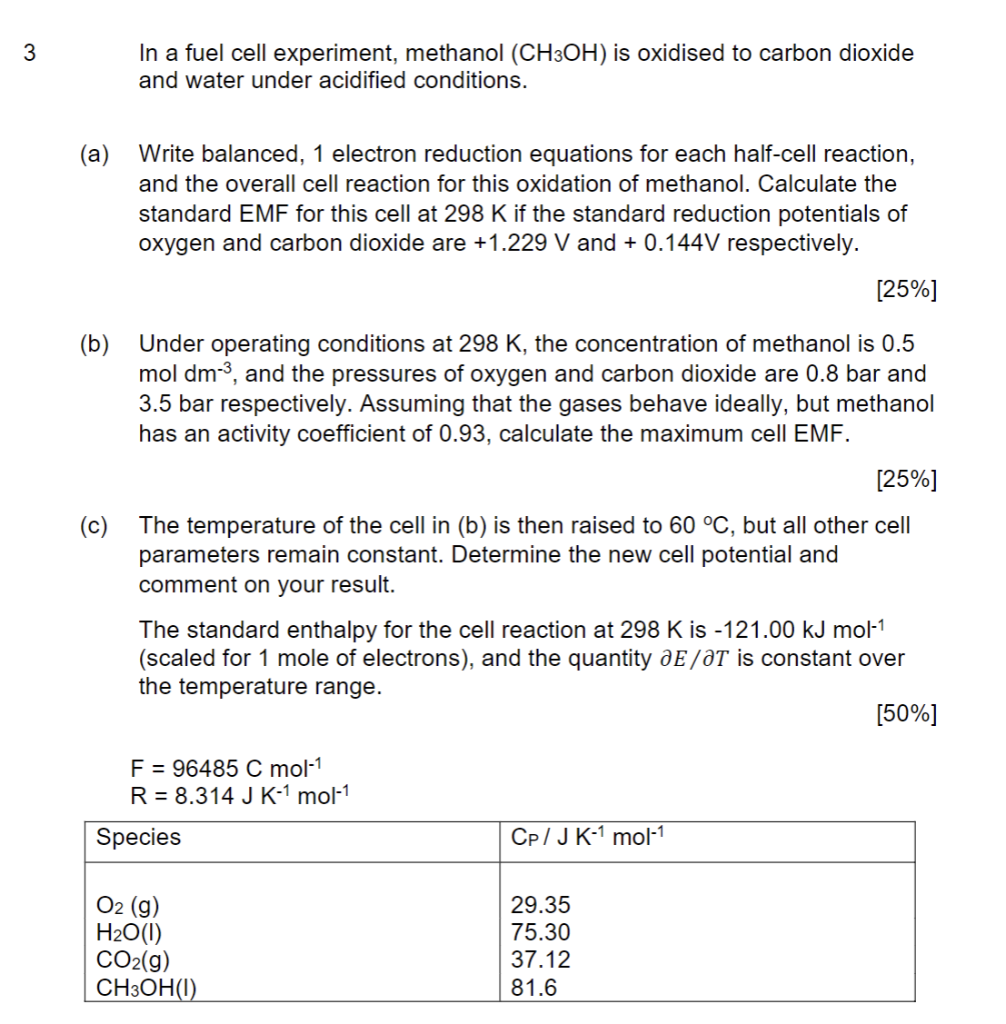
In a fuel cell experiment, methanol \( \left(\mathrm{CH}_{3} \mathrm{OH}\right) \) is oxidised to carbon dioxide and water under acidified conditions. (a) Write balanced, 1 electron reduction equations for each half-cell reaction, and the overall cell reaction for this oxidation of methanol. Calculate the standard EMF for this cell at \( 298 \mathrm{~K} \) if the standard reduction potentials of oxygen and carbon dioxide are \( +1.229 \mathrm{~V} \) and \( +0.144 \mathrm{~V} \) respectively. [25\%] (b) Under operating conditions at \( 298 \mathrm{~K} \), the concentration of methanol is \( 0.5 \) mol \( \mathrm{dm}^{-3} \), and the pressures of oxygen and carbon dioxide are \( 0.8 \) bar and \( 3.5 \) bar respectively. Assuming that the gases behave ideally, but methanol has an activity coefficient of \( 0.93 \), calculate the maximum cell EMF. [25\%] (c) The temperature of the cell in (b) is then raised to \( 60^{\circ} \mathrm{C} \), but all other cell parameters remain constant. Determine the new cell potential and comment on your result. The standard enthalpy for the cell reaction at \( 298 \mathrm{~K} \) is \( -121.00 \mathrm{~kJ} \mathrm{~mol}^{-1} \) (scaled for 1 mole of electrons), and the quantity \( \partial E / \partial T \) is constant over the temperature range. [50\%] \[ \begin{array}{l} \mathrm{F}=96485 \mathrm{C} \mathrm{mol} \mathrm{mol}^{-1} \\ \mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1} \end{array} \]
Expert Answer
Q. 3 Answer. A. The two half-cell reactions often occur at separate locations on the metal and, b