Home /
Expert Answers /
Chemistry /
imagine-you-have-a-mixture-of-these-two-refrigerants-at-160-circ-mathrm-c-and-2-math-pa195
(Solved): Imagine you have a mixture of these two refrigerants at \( 160^{\circ} \mathrm{C} \) and \( 2 \math ...
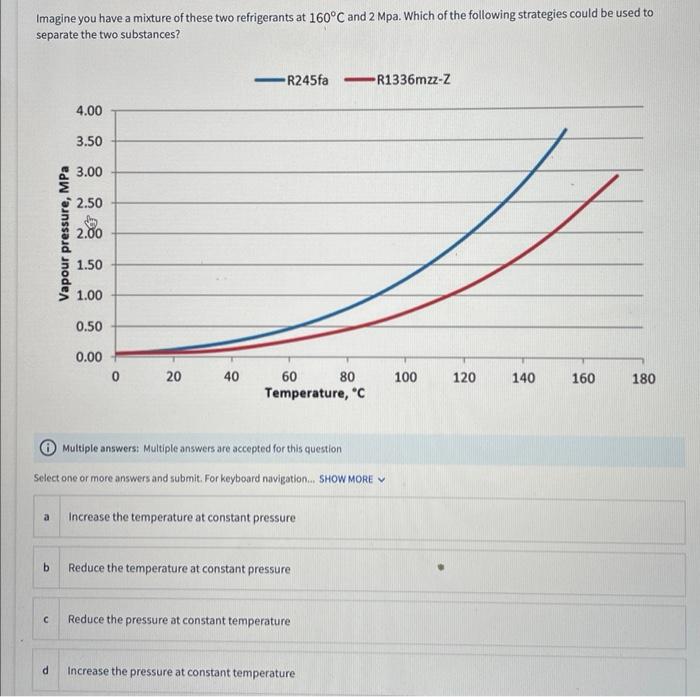
Imagine you have a mixture of these two refrigerants at \( 160^{\circ} \mathrm{C} \) and \( 2 \mathrm{Mpa} \). Which of the following strategies could be used to separate the two substances? (i) Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE \( \vee \) a Increase the temperature at constant pressure b Reduce the temperature at constant pressure c Reduce the pressure at constant temperature d Increase the pressure at constant temperature