Home /
Expert Answers /
Physics /
imagine-that-an-electron-in-an-excited-state-in-a-nitrogen-molecule-decays-to-iss-ground-state-emi-pa863
(Solved): Imagine that an electron in an excited state in a nitrogen molecule decays to iss ground state, emi ...
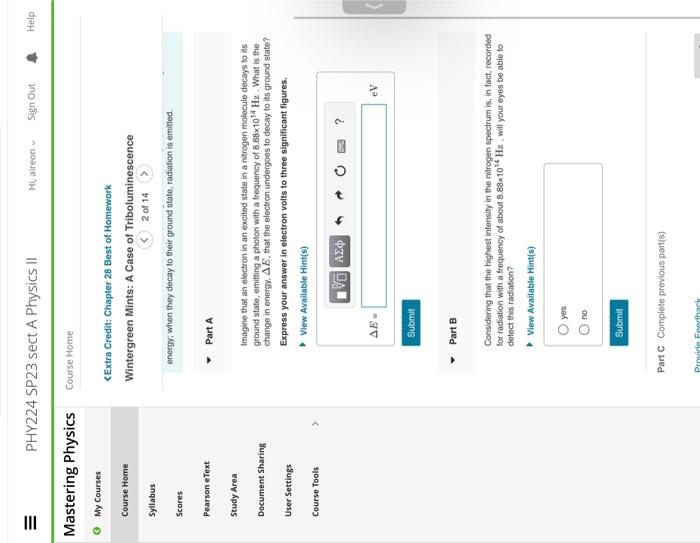
Imagine that an electron in an excited state in a nitrogen molecule decays to iss ground state, emitting a photon with a frequency of . What is the change in energy. , that the electron undergoes to decay to its ground state? Express your answer in electron volts to three significant figures. Part B Considering that the highest intensity in the nitrogen spectrum is, in fact, recorded for radiation with a frequency of about , will your eyes be able to detect this radiation?