Home /
Expert Answers /
Chemistry /
if-the-plot-is-not-displayed-please-see-figure-f-13-4-2-b-the-water-phase-diagram-in-the-e-pa221
(Solved): If the plot is not displayed, please see Figure \( F 13-4-2-b \) (the water phase diagram) in the e ...
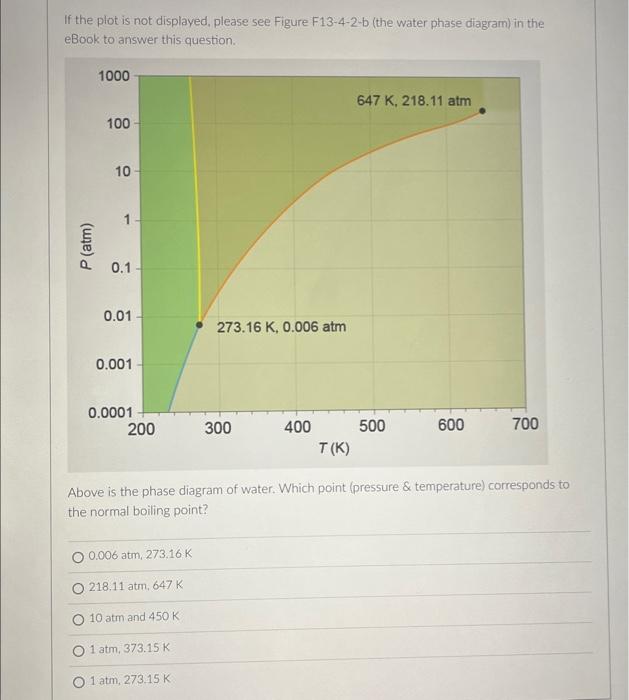
If the plot is not displayed, please see Figure \( F 13-4-2-b \) (the water phase diagram) in the eBook to answer this question. Above is the phase diagram of water. Which point (pressure \& temperature) corresponds to the normal boiling point? \( 0.006 \mathrm{~atm}_{,}, 273,16 \mathrm{~K} \) \( 218,11 \mathrm{~atm}, 647 \mathrm{~K} \) \( 10 \mathrm{~atm} \) and \( 450 \mathrm{~K} \) \( 1 \mathrm{~atm}, 373.15 \mathrm{~K} \) \( 1 \mathrm{~atm}, 273.15 \mathrm{~K} \)
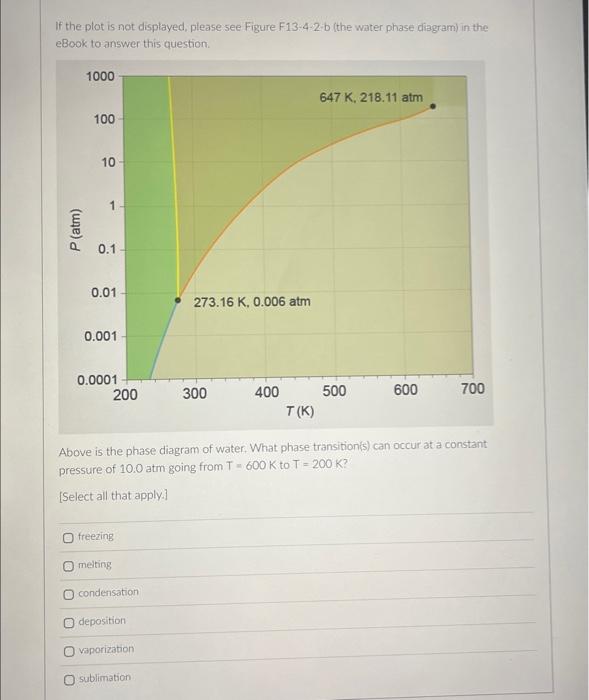
If the plot is not displayed, please see Figure \( F 13 \cdot 4 \cdot 2 \cdot b \) (the water phase diagram) in the eBook to answer this question. Above is the phase diagram of water. What phase transition(is) can occur at a constant pressure of \( 10.0 \mathrm{~atm} \) going from \( T=600 \mathrm{~K} \) to \( T=200 \mathrm{~K} \) ? [Select all that apply.] freezing melting condensation deposition vaporization sublimation
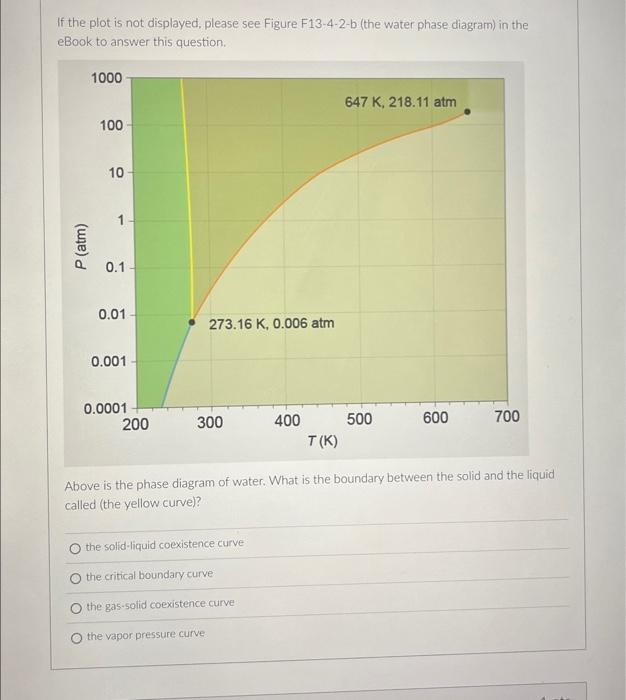
If the plot is not displayed, please see Figure F13-4-2-b (the water phase diagram) in the eBook to answer this question. Above is the phase diagram of water. What is the boundary between the solid and the liquid called (the yellow curve)? the solid-liquid coexistence curve the critical boundary curve the gas-solid coexistence curve the vapor pressure curve