Home /
Expert Answers /
Biology /
if-the-free-energy-of-converting-glucose-and-fructose-to-sucrose-dehydration-synthesis-is-27-pa326
(Solved): If the free energy of converting glucose and fructose to sucrose (dehydration synthesis) is \( 27 ...
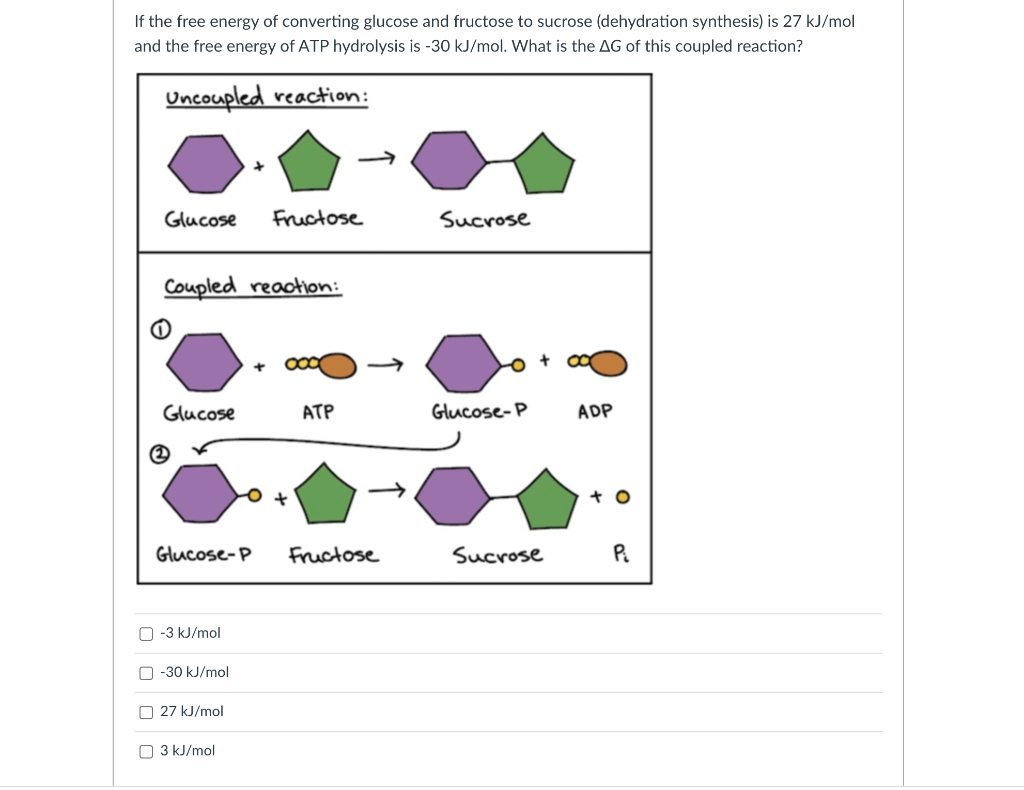
If the free energy of converting glucose and fructose to sucrose (dehydration synthesis) is \( 27 \mathrm{~kJ} / \mathrm{mol} \) and the free energy of ATP hydrolysis is \( -30 \mathrm{~kJ} / \mathrm{mol} \). What is the \( \Delta G \) of this coupled reaction? \( -3 \mathrm{~kJ} / \mathrm{mol} \) \( -30 \mathrm{~kJ} / \mathrm{mol} \) \( 27 \mathrm{~kJ} / \mathrm{mol} \) \( 3 \mathrm{~kJ} / \mathrm{mol} \)
Expert Answer
When the energy from the hydrolysis of ATP is utilized in driving other reactions then the reaction could be described as coupled reaction. In this wa