Home /
Expert Answers /
Chemistry /
identify-the-conjugate-base-of-phosphoric-acid-h3po4-o-hpo4-o-o-hpo4-hpo3-o-po4-ques-pa970
(Solved): Identify the conjugate base of phosphoric acid, H3PO4. O HPO4- O O HPO4 HPO3- O PO4- QUES ...
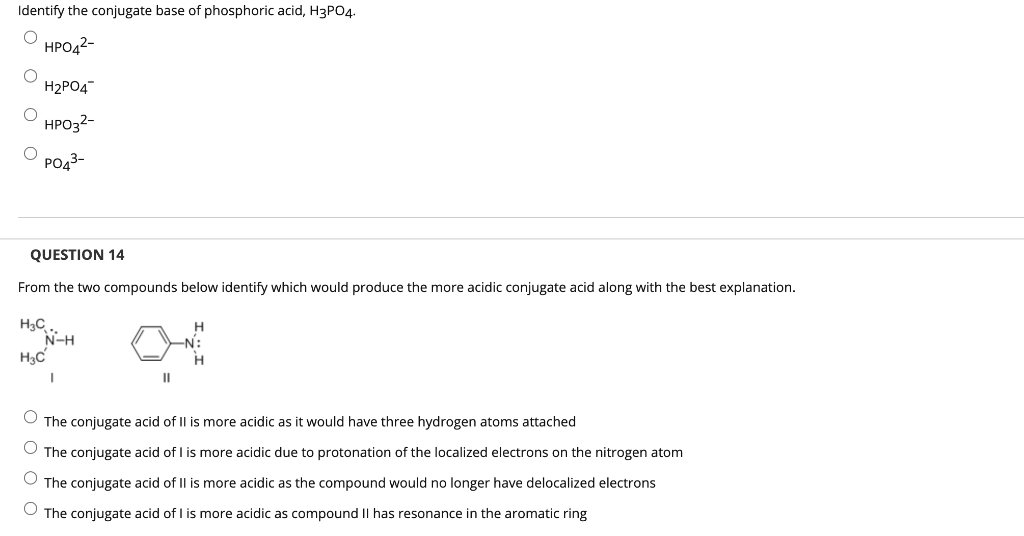
Identify the conjugate base of phosphoric acid, H3PO4. O HPO4²- O O H?PO4 HPO3²- O PO4³- QUESTION 14 From the two compounds below identify which would produce the more acidic conjugate acid along with the best explanation. H?C N-H H3C I II H O The conjugate acid of II is more acidic as it would have three hydrogen atoms attached O The conjugate acid of I is more acidic due to protonation of the localized electrons on the nitrogen atom The conjugate acid of II is more acidic as the compound would no longer have delocalized electrons O The conjugate acid of I is more acidic as compound II has resonance in the aromatic ring
Expert Answer
A)If H3PO4 or phosphoric acid looses a proton it will get converted to H2P