Home /
Expert Answers /
Chemistry /
identify-the-conjugate-base-for-each-acid-conjugate-base-of-mathrm-h-3-mathrm-po-4-pa157
(Solved): Identify the conjugate base for each acid. conjugate base of \( \mathrm{H}_{3} \mathrm{PO}_{4} \) : ...
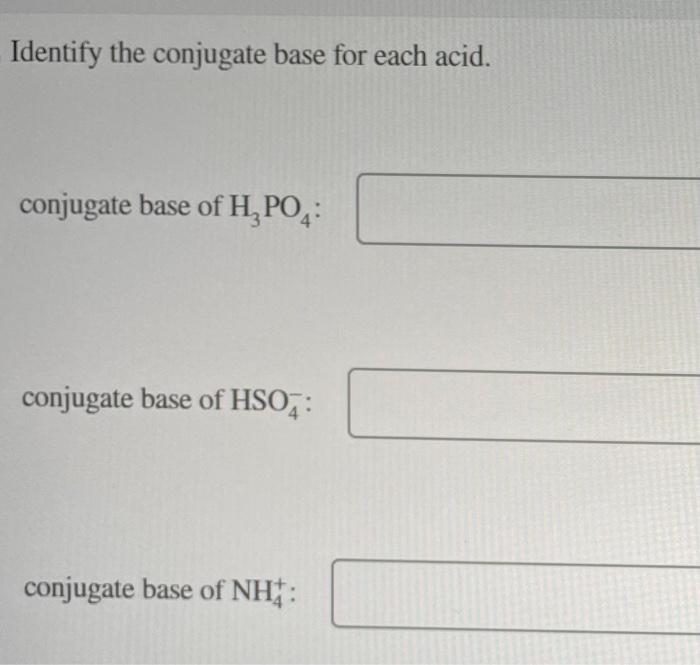


Identify the conjugate base for each acid. conjugate base of \( \mathrm{H}_{3} \mathrm{PO}_{4} \) : conjugate base of \( \mathrm{HSO}_{4}^{-} \): conjugate base of \( \mathrm{NH}_{4}^{+} \):
Complete and balance the equations according to acid and base behavior in water. Phases are optional. \[ \mathrm{HNO}_{3}(\mathrm{aq}) \stackrel{\mathrm{H}_{2} \mathrm{O}}{\longrightarrow} \] \[ \mathrm{Ba}(\mathrm{OH})_{2}(\mathrm{~s}) \stackrel{\mathrm{H}_{2} \mathrm{O}}{\longrightarrow} \] \[ \mathrm{HF}(\mathrm{aq}) \stackrel{\mathrm{H}_{2} \mathrm{O}}{\rightleftharpoons} \]
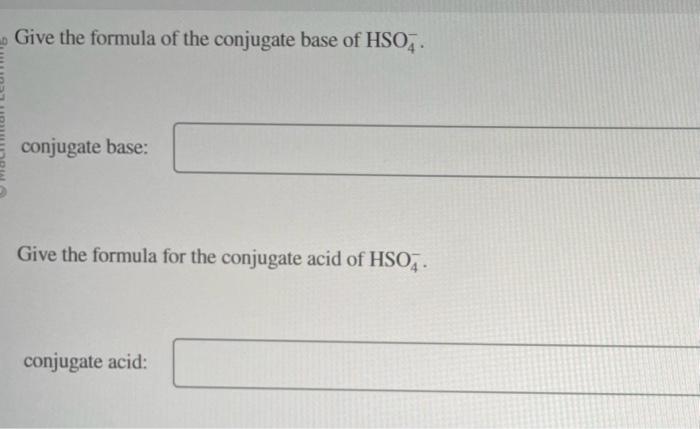
Give the formula of the conjugate base of \( \mathrm{HSO}_{4}^{-} \). conjugate base: Give the formula for the conjugate acid of \( \mathrm{HSO}_{4}^{-} \). conjugate acid:
Calculate the \( \mathrm{pH} \) for each \( \mathrm{H}_{3} \mathrm{O}^{+} \)concentration. \[ \left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=1 \times 10^{-8} \mathrm{M} \] \[ \mathrm{pH}= \] \[ \left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=0.01 \mathrm{M} \] \[ \mathrm{pH}= \] \[ \left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=1 \times 10^{-13} \mathrm{M} \]