Home /
Expert Answers /
Chemistry /
identify-each-of-the-following-reactions-as-being-a-neutralization-precipitation-or-oxidationredu-pa564
(Solved): Identify each of the following reactions as being a neutralization, precipitation, or oxidationredu ...
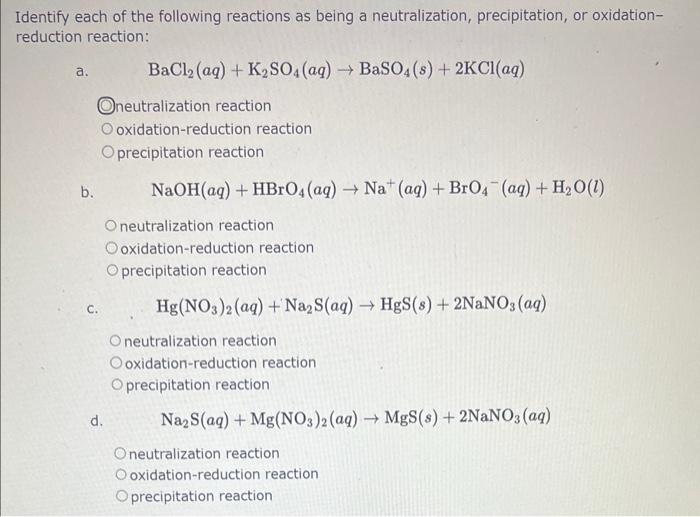
Identify each of the following reactions as being a neutralization, precipitation, or oxidationreduction reaction: a. \( \quad \mathrm{BaCl}_{2}(a q)+\mathrm{K}_{2} \mathrm{SO}_{4}(a q) \rightarrow \mathrm{BaSO}_{4}(s)+2 \mathrm{KCl}(a q) \) neutralization reaction oxidation-reduction reaction precipitation reaction b. \( \mathrm{NaOH}(a q)+\mathrm{HBrO}_{4}(a q) \rightarrow \mathrm{Na}^{+}(a q)+\mathrm{BrO}_{4}^{-}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \) neutralization reaction oxidation-reduction reaction precipitation reaction c. \( \mathrm{Hg}\left(\mathrm{NO}_{3}\right)_{2}(a q)+\mathrm{Na}_{2} \mathrm{~S}(a q) \rightarrow \mathrm{HgS}(s)+2 \mathrm{NaNO}_{3}(a q) \) neutralization reaction oxidation-reduction reaction precipitation reaction d. \( \mathrm{Na}_{2} \mathrm{~S}(a q)+\mathrm{Mg}\left(\mathrm{NO}_{3}\right)_{2}(a q) \rightarrow \mathrm{MgS}(s)+2 \mathrm{NaNO}_{3}(a q) \) neutralization reaction oxidation-reduction reaction precipitation reaction
Expert Answer
Reaction 1: The aquous solution of BaCl2 and K2SO4 are forming a precipitate BaSO4, therefore the reaction is a Precipit