Home /
Expert Answers /
Chemistry /
i-need-help-with-these-3-problems-the-activation-energy-ea-for-a-particular-reaction-is-42-2kj-mol-pa602
(Solved): i need help with these 3 problems The activation energy Ea for a particular reaction is 42.2kJ/mol ...
i need help with these 3 problems
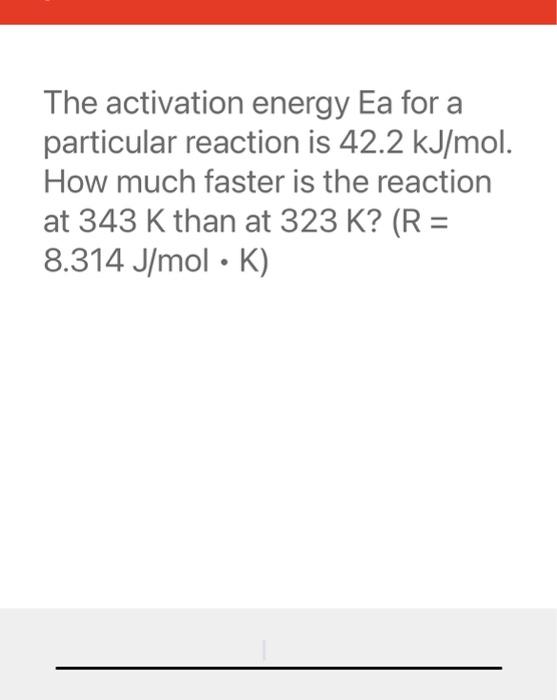





The activation energy Ea for a particular reaction is . How much faster is the reaction at than at ?
The activation energy for a particular reaction is . If the rate constant is at , what is the rate constant at ?
The kinetics of a gas phase reaction of the form Products results in a rate constant of . For this reaction, the initial concentration of is . What is the half-life for this reaction?