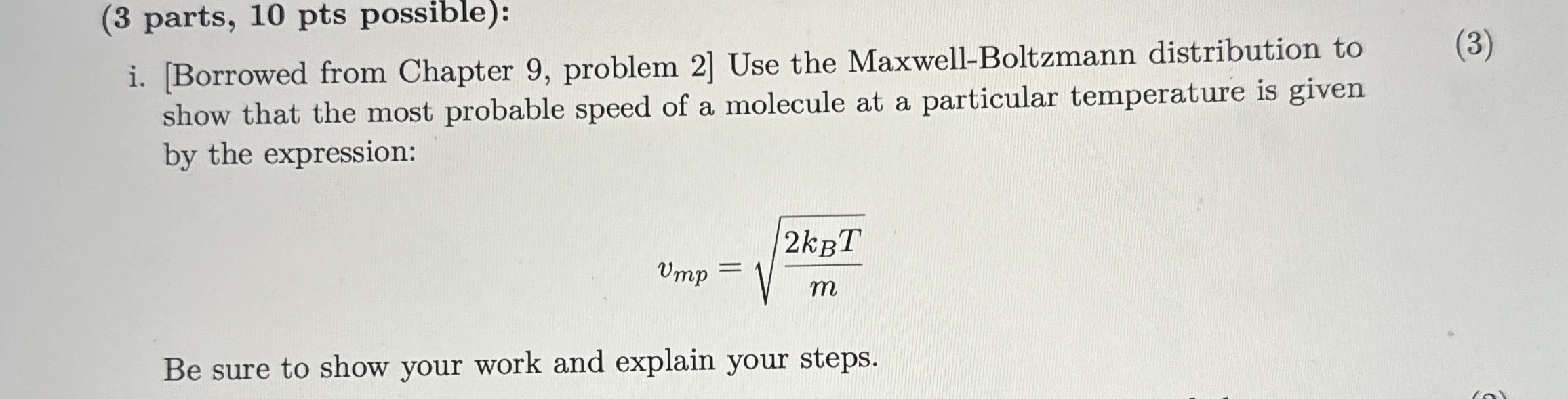Home /
Expert Answers /
Advanced Physics /
i-borrowed-from-chapter-9-problem-2-use-the-maxwell-boltzmann-distribution-to-show-that-the-mos-pa810
(Solved): i. [Borrowed from Chapter 9, problem 2] Use the Maxwell-Boltzmann distribution to show that the mos ...
i. [Borrowed from Chapter 9, problem 2] Use the Maxwell-Boltzmann distribution to show that the most probable speed of a molecule at a particular temperature is given by the expression:
v_(mp)=\sqrt((2k_(B)T)/(m))Be sure to show your work and explain your steps. (3 parts, 10 pts possible): i. [Borrowed from Chapter 9, problem 2] Use the Maxwell-Boltzmann distribution to show that the most probable speed of a molecule at a particular temperature is given by the expression:
v_(mp)=\sqrt((2k_(B)T)/(m))Be sure to show your work and explain your steps.