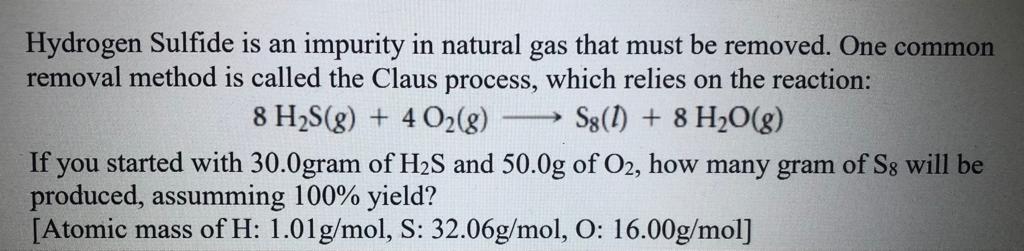Home /
Expert Answers /
Chemical Engineering /
hydrogen-sulfide-is-an-impurity-in-natural-gas-that-must-be-removed-one-common-removal-method-is-pa416
(Solved): Hydrogen Sulfide is an impurity in natural gas that must be removed. One common removal method is ...
Hydrogen Sulfide is an impurity in natural gas that must be removed. One common removal method is called the Claus process, which relies on the reaction: \[ 8 \mathrm{H}_{2} \mathrm{~S}(g)+4 \mathrm{O}_{2}(g) \longrightarrow \mathrm{S}_{8}(l)+8 \mathrm{H}_{2} \mathrm{O}(g) \] If you started with \( 30.0 \) gram of \( \mathrm{H}_{2} \mathrm{~S} \) and \( 50.0 \mathrm{~g} \) of \( \mathrm{O}_{2} \), how many gram of \( \mathrm{S}_{8} \) will be produced, assumming \( 100 \% \) yield? [Atomic mass of \( \mathrm{H}: 1.01 \mathrm{~g} / \mathrm{mol}, \mathrm{S}: 32.06 \mathrm{~g} / \mathrm{mol}, \mathrm{O}: 16.00 \mathrm{~g} / \mathrm{mol}] \)