Home /
Expert Answers /
Chemistry /
hydrogen-peroxide-decomposes-to-water-and-oxygen-at-constant-pressure-by-the-following-reaction-2h-pa614
(Solved): Hydrogen peroxide decomposes to water and oxygen at constant pressure by the following reaction: 2H ...
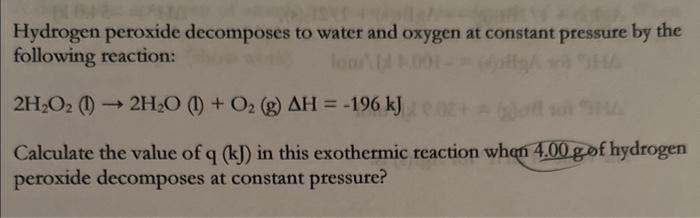
Hydrogen peroxide decomposes to water and oxygen at constant pressure by the following reaction: Calculate the value of in this exothermic reaction when hydrogen peroxide decomposes at constant pressure?
Expert Answer
As it is given that the change of enthalpy of 2 moles of