Home /
Expert Answers /
Chemistry /
hybridization-is-used-to-explain-not-only-the-number-of-bonds-formed-in-a-compound-but-the-equival-pa534
(Solved): Hybridization is used to explain not only the number of bonds formed in a compound but the equival ...
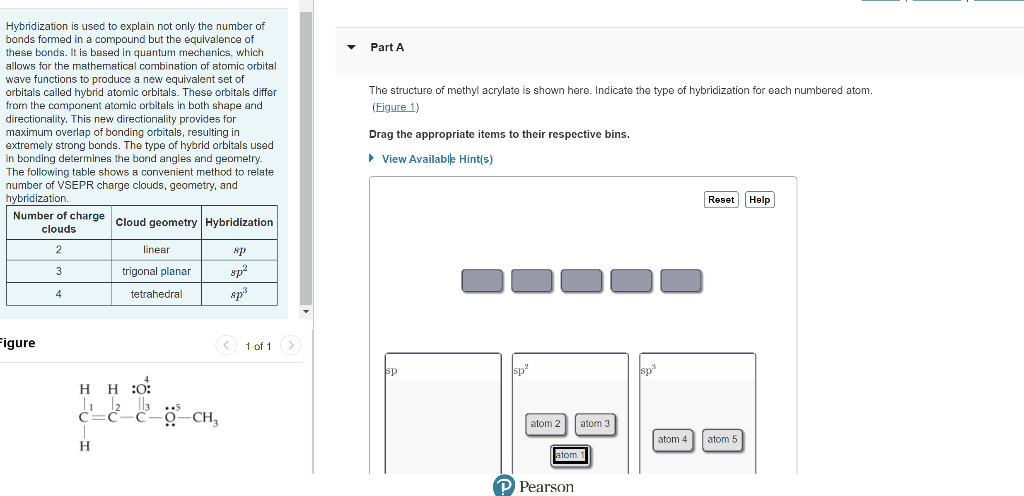
Hybridization is used to explain not only the number of bonds formed in a compound but the equivalence of these bonds. It is besed in quantum mechanics, which allows for the mathematical combination of atomic orbital wave functions to produce a new equivalent set of orbitals called hybrid atomic orbitals. These orbitals differ The structure of methyl acrylate is shown here. Indicate the type of hybridization for each numbered atom. from the component atomic orbitals in both shape and (Eigure 1) directionality. This new directionality provides for maximum overlap of bonding orbitals, resulting in Drag the appropriate items to their respective bins. extremely strong bonds. The type of hybrid orbitals used in bonding determines the bond angles and geometry. The following table shows a convenient method to relate number of VSEPR charge clouds, geometry, and hvbridization rigure 1 of 1