Home /
Expert Answers /
Chemistry /
how-will-the-volume-of-a-fixed-sample-of-gas-change-if-its-pressure-is-doubled-and-the-kelvin-tempe-pa412
(Solved): How will the volume of a fixed sample of gas change if its pressure is doubled and the Kelvin tempe ...
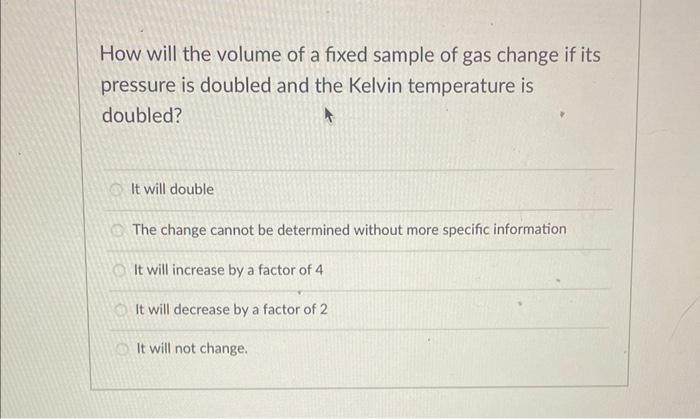
How will the volume of a fixed sample of gas change if its pressure is doubled and the Kelvin temperature is doubled? It will double The change cannot be determined without more specific information It will increase by a factor of 4 It will decrease by a factor of 2 It will not change.
Expert Answer
According to equation PV=nRT (P = pressure , V= volume , n= moles of gas , R is gas constant and T is tem