Home /
Expert Answers /
Chemistry /
how-do-you-calculate-the-concentration-of-the-buffer-the-buffer-is-made-up-of-25cm3-nbsp-of-0-8m-ch-pa245
(Solved): How do you calculate the concentration of the buffer? The buffer is made up of 25cm3 of 0,8M CH ...
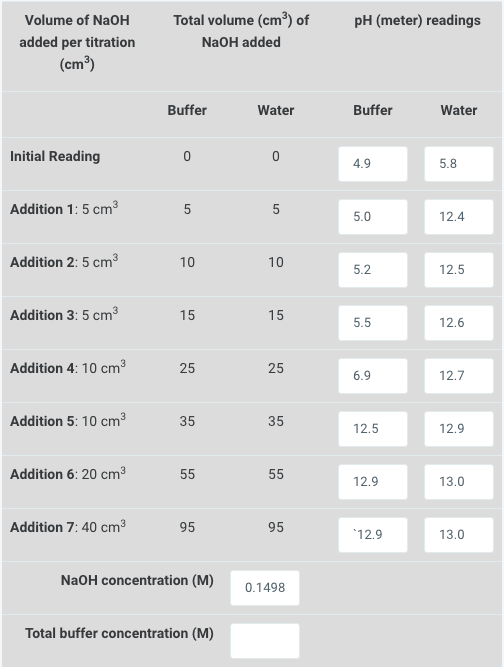
How do you calculate the concentration of the buffer?
The buffer is made up of 25cm3 of 0,8M CH3COONa-solution and 10cm3 of 2M CH3COOH-solution, diluted with distilled water until 200cm3.
\( \begin{array}{ccc}\begin{array}{c}\text { Volume of } \mathrm{NaOH} \\ \text { added per titration }\end{array} & \text { Total volume }\left(\mathrm{cm}^{3}\right) \text { of } & \mathrm{pH} \text { (meter) readings } \\ \left(\mathrm{cm}^{3}\right) & \mathrm{NaOH} \text { added }\end{array} \)
Expert Answer
M3 = 0.2 M Explanation: The buffer concentration can be calculated using the equation: M1V1 + M2V2 = M3V3