Home /
Expert Answers /
Chemistry /
how-do-i-find-c-agno3-and-the-final-results-atomic-weight-of-ag-atomic-weight-of-i-mass-of-unkn-pa942
(Solved): How do I find c(AgNO3) and the final results? Atomic weight of Ag Atomic weight of I Mass of unkn ...
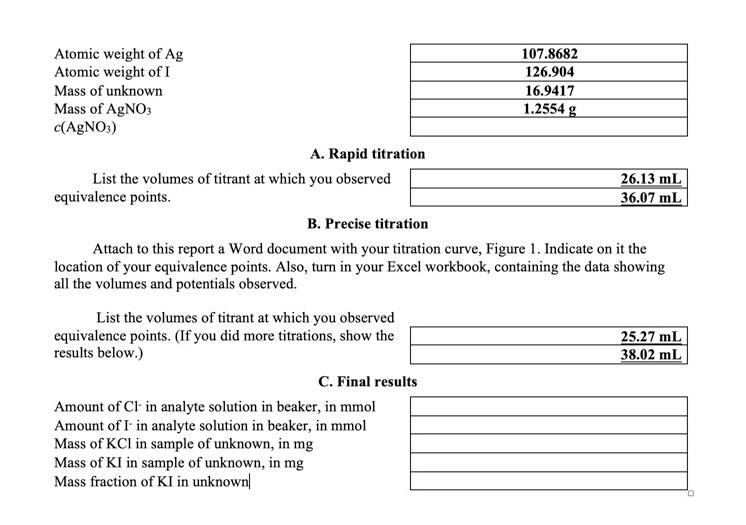
How do I find c(AgNO3) and the final results?
Atomic weight of Atomic weight of I Mass of unknown Mass of A. Rapid titration List the volumes of titrant at which you observed equivalence points. B. Precise titration Attach to this report a Word document with your titration curve, Figure 1. Indicate on it the location of your equivalence points. Also, turn in your Excel workbook, containing the data showing all the volumes and potentials observed. List the volumes of titrant at which you observed equivalence points. (If you did more titrations, show the results below.) C. Final results Amount of in analyte solution in beaker, in mmol Amount of in analyte solution in beaker, in mmol Mass of in sample of unknown, in Mass of KI in sample of unknown, in mg Mass fraction of KI in unknown|
Expert Answer
Moles of any compound is calculated as mass of compound divided by molar mass of compoundExplanationMath