Home /
Expert Answers /
Chemistry /
how-can-i-find-mw-of-the-salt-and-atomic-mass-nbsp-identification-of-the-unknown-alkali-carbonat-pa713
(Solved): how can i find mw of the salt and atomic mass Identification of the Unknown Alkali Carbonat ...
how can i find mw of the salt and atomic mass
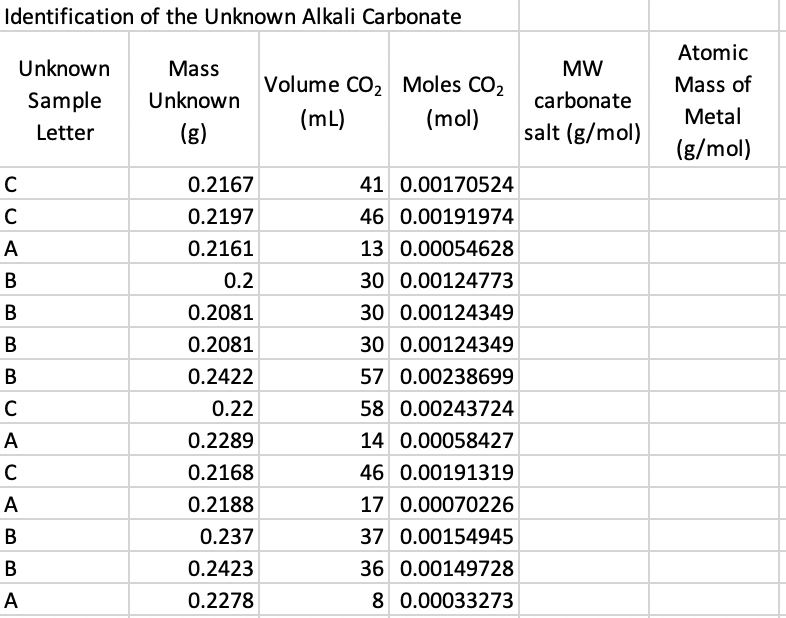
Identification of the Unknown Alkali Carbonate \begin{tabular}{|l|r|r|r|r|c|} \hline Unknown Sample Letter & Mass Unknown (g) & Volume \( \mathrm{CO}_{2} \) \( (\mathrm{~mL}) \) & Moles \( \mathrm{CO}_{2} \) (mol) & MW carbonate salt \( (\mathrm{g} / \mathrm{mol}) \) & Atomic Mass of Metal \( (\mathrm{g} / \mathrm{mol}) \) \\ \hline C & \( 0.2167 \) & 41 & \( 0.00170524 \) & & \\ \hline C & \( 0.2197 \) & 46 & \( 0.00191974 \) & & \\ \hline A & \( 0.2161 \) & 13 & \( 0.00054628 \) & & \\ \hline B & \( 0.2 \) & 30 & \( 0.00124773 \) & \\ \hline B & \( 0.2081 \) & 30 & \( 0.00124349 \) & \\ \hline B & \( 0.2081 \) & 30 & \( 0.00124349 \) & \\ \hline B & \( 0.2422 \) & 57 & \( 0.00238699 \) & \\ \hline C & \( 0.22 \) & 58 & \( 0.00243724 \) & \\ \hline A & \( 0.2289 \) & 14 & \( 0.00058427 \) & \\ \hline C & \( 0.2168 \) & 46 & \( 0.00191319 \) & \\ \hline B & \( 0.2188 \) & 17 & \( 0.00070226 \) & \\ \hline B & \( 0.237 \) & 37 & \( 0.00154945 \) & \\ \hline A & \( 0.2423 \) & 36 & \( 0.00149728 \) & \\ \hline \end{tabular}
Expert Answer
Ans - Alkali metals are the metals of Group - 1 of the modern periodic table. These are Lithium, Sodium, Potassium, Rubidium, Caesium and Francium. Now the formula for their c