Home /
Expert Answers /
Chemistry /
help-plsase-answer-a-e-thank-you-consider-the-following-acids-and-their-dissociation-constants-2-pa483
(Solved): help, plsase answer A-E thank you Consider the following acids and their dissociation constants: 2- ...
help, plsase answer A-E thank you
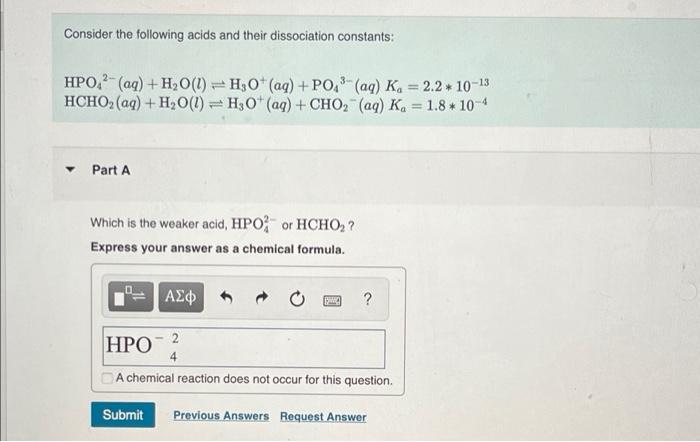
Consider the following acids and their dissociation constants: 2- HPO4 (aq) + H?O(1) HCHO? (aq) + H?O(1) Part A T | ??? T H3O+ (aq) + PO4³ (aq) K?= 2.2 10-13 H3O+ (aq) + CHO? (aq) Ka 1.8*10-4 Which is the weaker acid, HPO or HCHO?? Express your answer as a chemical formula. BINO ? HPO 4 A chemical reaction does not occur for this question. Submit Previous Answers Request Answer
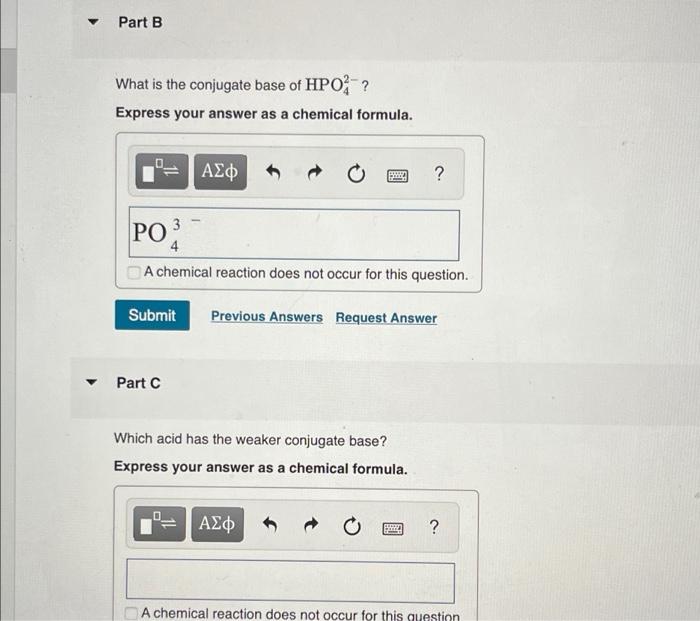
Part B What is the conjugate base of HPO?? Express your answer as a chemical formula. PO ??? Part C BOD BARRY 4 A chemical reaction does not occur for this question. Submit Previous Answers Request Answer Which acid has the weaker conjugate base? Express your answer as a chemical formula. ??? ? ? A chemical reaction does not occur for this question
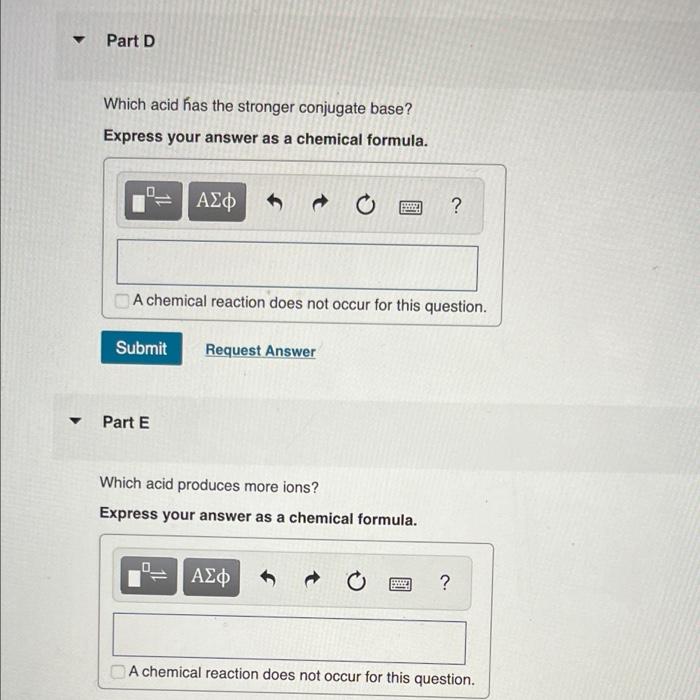
? Part D Which acid has the stronger conjugate base? Express your answer as a chemical formula. 0_ = | ??? Submit Part E A chemical reaction does not occur for this question. ? 0 Request Answer www Which acid produces more ions? Express your answer as a chemical formula. ??? ? ? A chemical reaction does not occur for this question.