Home /
Expert Answers /
Chemistry /
help-please-use-the-information-in-the-aleks-data-tab-to-sort-the-following-chemical-species-by-pa896
(Solved): HELP PLEASE!!!!! Use the information in the ALEKS Data tab to sort the following chemical species by ...
HELP PLEASE!!!!!
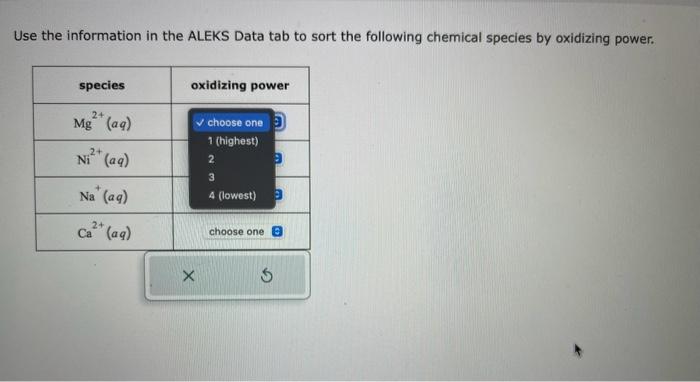

Use the information in the ALEKS Data tab to sort the following chemical species by oxidizing power.
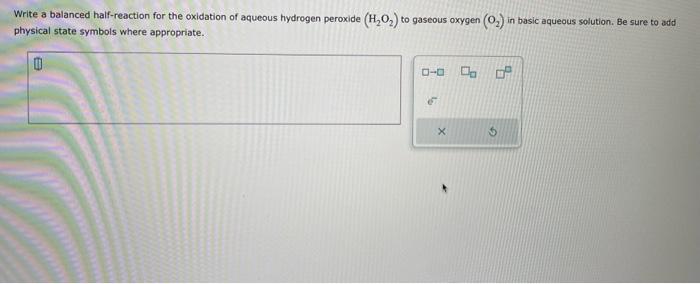
Write a balanced half-reaction for the oxidation of aqueous hydrogen peroxide \( \left(\mathrm{H}_{2} \mathrm{O}_{2}\right) \) to gaseous oxygen \( \left(\mathrm{O}_{2}\right) \) in basic aqueous solution. Be sure to add physical state symbols where appropriate.
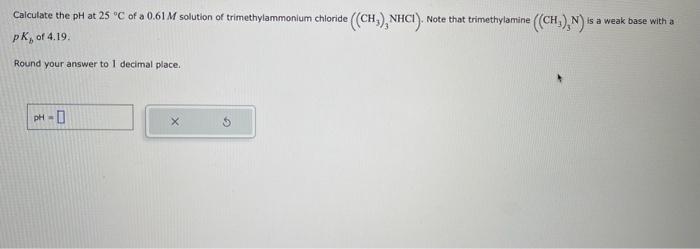
Caiculate the \( \mathrm{pH} \) at \( 25^{-\circ} \mathrm{C} \) of a \( 0.61 \mathrm{M} \) solution of trimethylammonium chloride \( \left(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{NHCl}\right) \). Note that trimethylamine \( \left(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}\right) \) is a weak base with a \( p K_{b} \) of \( 4.19 \) Round your answer to l decimal place.
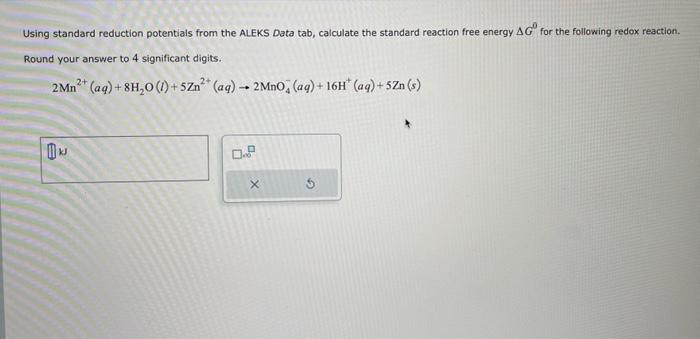
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy \( \Delta G^{0} \) for the following redox reaction. Round your answer to 4 significant digits. \[ 2 \mathrm{Mn}^{2+}(a q)+8 \mathrm{H}_{2} \mathrm{O}(l)+5 \mathrm{Zn}^{2+}(a q) \rightarrow 2 \mathrm{MnO}_{4}^{-}(a q)+16 \mathrm{H}^{+}(a q)+5 \mathrm{Zn}(s) \]
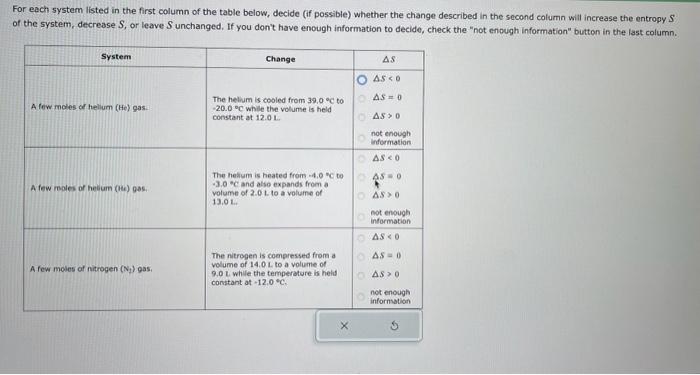
For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy \( S \) of the system, decrease \( S \), or leave \( S \) unchanged, If you don't have enough information to decide, check the "not enough information" button in the last column.
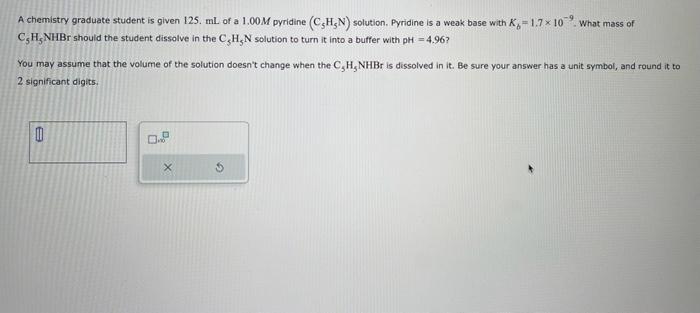
A chemistry graduate student is given 125. \( \mathrm{mL} \) of a \( 1.00 \mathrm{M} \) pyridine \( \left(\mathrm{C}_{5} \mathrm{H}_{5} \mathrm{~N}\right) \) solution. Pyridine is a weak base with \( K_{b}=1.7 \times 10 \). What mass of \( \mathrm{C}_{5} \mathrm{H}_{5} \mathrm{NHBr} \) should the student dissolve in the \( \mathrm{C}_{5} \mathrm{H}_{5} \mathrm{~N} \) solution to turn it into a buffer with \( \mathrm{pH}=4.96 \) ? You may assume that the volume of the solution doesn't change when the \( \mathrm{C}_{5} \mathrm{H}_{5} \mathrm{NHBr} \) is dissolved in it. Be sure your answer has a unit symbol, and round it 2 significant digits.