Home /
Expert Answers /
Chemical Engineering /
he-liquid-phase-reaction-triphenyl-methyl-chloride-a-methanol-b-products-c-hcl-was-arried-pa370
(Solved): he liquid-phase reaction Triphenyl methyl chloride (A)+ Methanol (B) Products (C)+HCl was arried ...
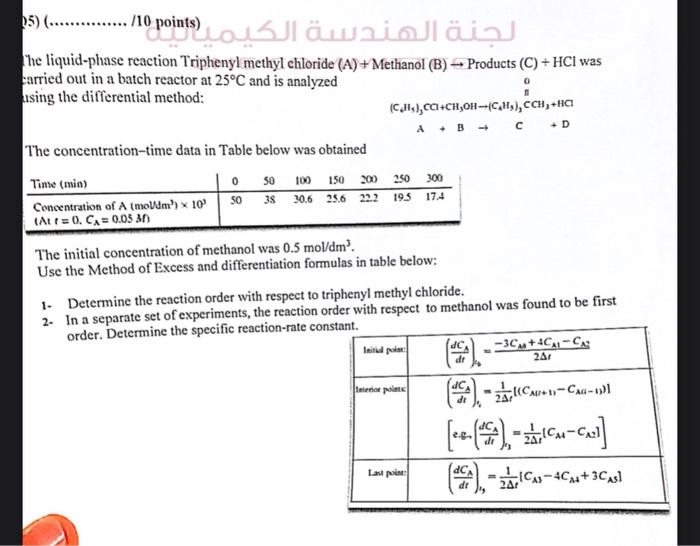
he liquid-phase reaction Triphenyl methyl chloride Methanol Products was arried out in a batch reactor at and is analyzed ising the differential method: The concentration-time data in Table below was obtained The initial concentration of methanol was . Use the Method of Excess and differentiation formulas in table below: 1- Determine the reaction order with respect to triphenyl methyl chloride. 2. In a separate set of experiments, the reaction order with respect to methanol was found to be first order. Determine the specific reaction-rate constant.