Home /
Expert Answers /
Chemistry /
half-reactions-identifying-oxidation-reductionfor-the-following-equation-and-they-should-be-balanced-pa180
(Solved): half reactions(identifying oxidation/reductionfor the following equation(and they should be balanced ...
half reactions(identifying oxidation/reductionfor the following equation(and they should be balanced)
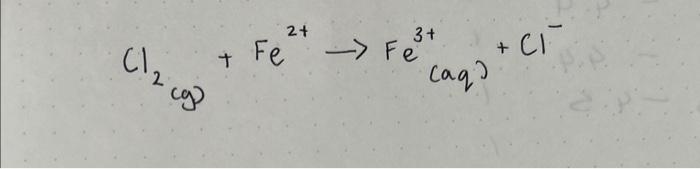
\( \left.\mathrm{Cl}_{2(g)}+\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}_{(\mathrm{aq}}\right)^{3+}+\mathrm{Cl}^{-} \)
Expert Answer
Cl2+Fe2+ ?Fe3++Cl-(0)(+2)(+3)(-1)Reductio