Home /
Expert Answers /
Chemistry /
half-lifo-equation-for-first-order-reactione-t-1-2-frac-0-093-k-where-t-1-2-pa958
(Solved): Half-lifo equation for first-order reactione: \[ t_{1 / 2}=\frac{0.093}{k} \] where \( t_{1 / 2} \) ...
![Half-lifo equation for first-order reactione:
\[
t_{1 / 2}=\frac{0.093}{k}
\]
where \( t_{1 / 2} \) is the half-life in secon](https://media.cheggcdn.com/study/93d/93dc9f0c-ed7d-400e-975b-1517993e579f/image)
Half-lifo equation for first-order reactione: \[ t_{1 / 2}=\frac{0.093}{k} \] where \( t_{1 / 2} \) is the half-life in seconds \( (s) \), and \( k \) is the rate constant in inverse seconds \( \left(\mathrm{s}^{-1}\right) \). Part A To calculate the half-life, plug the value for \( k \) into the half-life equation and solve. What is the half-life of a first-order reaction with a rate constant of \( 8.80 \times 10^{-4} \mathrm{~s}^{-1} \) ? Express your answer with the appropriate units.
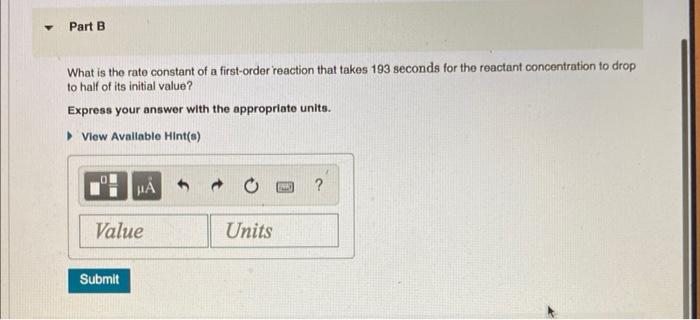
What is the rate constant of a first-order reaction that takes 193 seconds for the reactant concentration to drop to half of its initial value? Express your answer with the appropriate units.
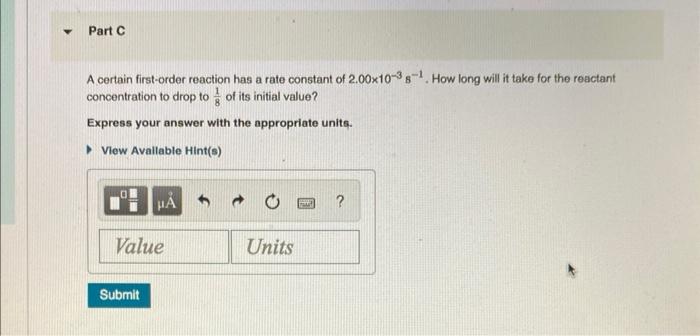
A certain first-order reaction has a rate constant of \( 2.00 \times 10^{-3} \mathrm{~s}^{-1} \). How long will it take for the reactant concentration to drop to \( \frac{1}{8} \) of its initial value? Express your answer with the appropriate units.