Home /
Expert Answers /
Chemistry /
guanidine-is-a-stronger-base-than-the-typical-amine-the-increased-basicity-can-be-explained-by-dra-pa324
(Solved): Guanidine is a stronger base than the typical amine. The increased basicity can be explained by dra ...

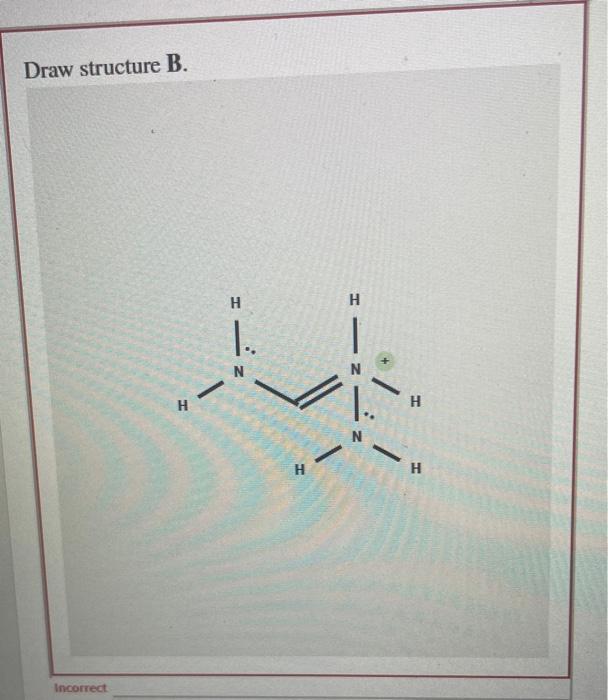
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by drawing the resonance structures of the protonated guanidine. The protonated guanidine has been drawn for you. Draw major resonance structures, and , and one minor resonance structure, . Be sure to include the formal charge, lone pairs, and hydrogens on nitrogen for structures B, C, and D.
Draw structure B.
Expert Answer
general rules for drawing resonance structures:The position of atoms of the molecules are not changed while drawing resonance forms.While drawing reso