Home /
Expert Answers /
Chemistry /
given-the-two-reactions-3-pbcl2-aq-pb2-aq-2cl-aq-k3-1-801010-and-4-agcl-aq-pa714
Expert Answer
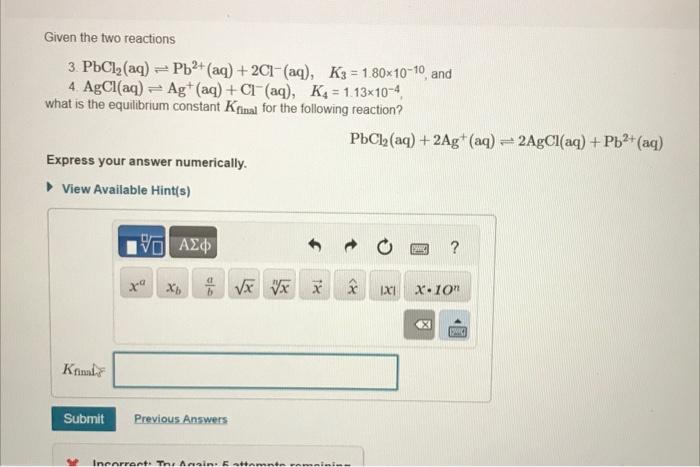
Given that PbClA2(aq)????PbA2+(aq)+2ClA?(aq) KA3= [P