Home /
Expert Answers /
Chemistry /
given-the-tlc-thin-layer-chromatography-plate-below-determine-the-approximate-mathbf-r-mat-pa593
(Solved): Given the TLC (thin layer chromatography) plate below determine the approximate \( \mathbf{R}_{\mat ...
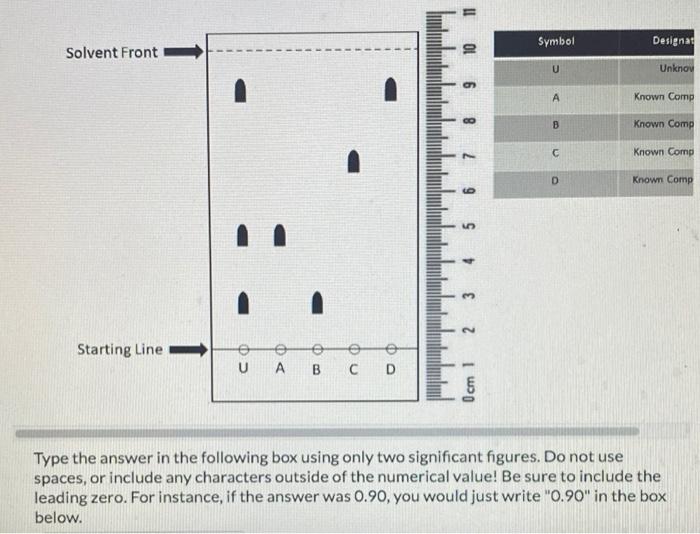
Given the TLC (thin layer chromatography) plate below determine the approximate \( \mathbf{R}_{\mathbf{f}} \) value for "Known Compound C." - The following TLC plate has been fully developed and marked so that all spots are indicated. - The stationary phase of the TLC plate is composed of relatively polar silica gel. In other words, a standard stationary phase is being used. - The "starting line" represents where a given compound or multiple compounds have been spotted before development of the TLC plate (see key). - The "solvent front" represents the distance traveled by the eluent (solvent) up the TLC plate.
Type the answer in the following box using only two significant figures. Do not use spaces, or include any characters outside of the numerical value! Be sure to include the leading zero. For instance, if the answer was \( 0.90 \), you would just write " \( 0.90 \) " in the box below.
Expert Answer
Rf value is the ratio of the distance travelled by solute to the distance t