Home /
Expert Answers /
Chemistry /
given-the-following-information-formic-acid-hcooh-hydrocyanic-acid-hcn-hcooh-is-a-stronger-acid-pa494
(Solved): Given the following information: formic acid =HCOOH hydrocyanic acid =HCN HCOOH is a stronger acid ...
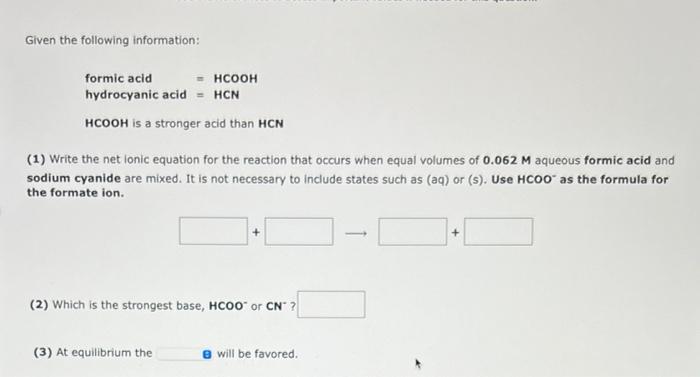
Given the following information: formic acid hydrocyanic acid is a stronger acid than (1) Write the net Ionic equation for the reaction that occurs when equal volumes of aqueous formic acid and sodium cyanide are mixed. It is not necessary to include states such as (aq) or (s). Use as the formula for the formate ion. (2) Which is the strongest base, or ? (3) At equilibrium the will be favored.