Home /
Expert Answers /
Chemistry /
given-that-the-solubility-reaction-for-calcium-phosphate-is-ca3-po4-2-s-3ca2-aq-2po-pa795
(Solved): Given that the solubility reaction for calcium phosphate is Ca3(PO4)2(s)3Ca2+(aq)+2PO ...
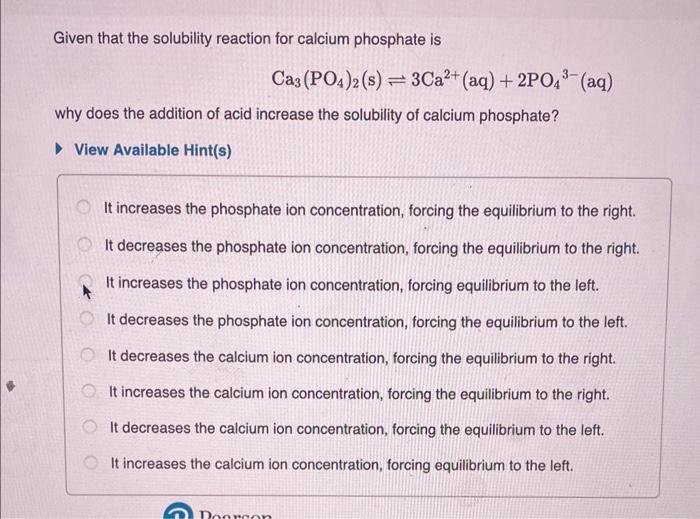
Given that the solubility reaction for calcium phosphate is why does the addition of acid increase the solubility of calcium phosphate? View Available Hint(s) It increases the phosphate ion concentration, forcing the equilibrium to the right. It decreases the phosphate ion concentration, forcing the equilibrium to the right. It increases the phosphate ion concentration, forcing equilibrium to the left. It decreases the phosphate ion concentration, forcing the equilibrium to the left. It decreases the calcium ion concentration, forcing the equilibrium to the right. It increases the calcium ion concentration, forcing the equilibrium to the right. It decreases the calcium ion concentration, forcing the equilibrium to the left. It increases the calcium ion concentration, forcing equilibrium to the left.
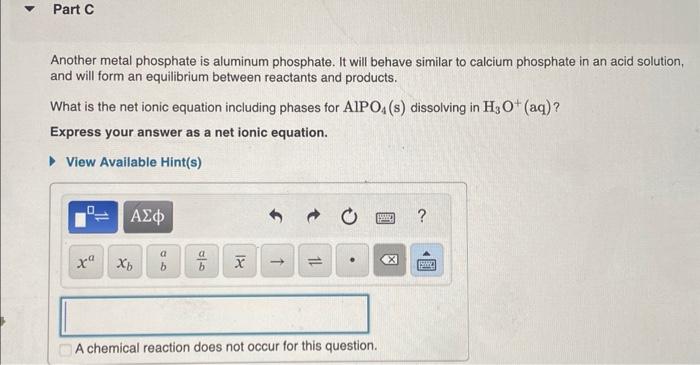
Another metal phosphate is aluminum phosphate. It will behave similar to calcium phosphate in an acid solution, and will form an equilibrium between reactants and products. What is the net ionic equation including phases for dissolving in (aq)? Express your answer as a net ionic equation.