Home /
Expert Answers /
Chemistry /
from-the-following-heats-of-combustion-calculate-the-enthalpy-of-formation-of-methanol-left-m-pa250
(Solved): From the following heats of combustion, calculate the enthalpy of formation of methanol \( \left(\m ...
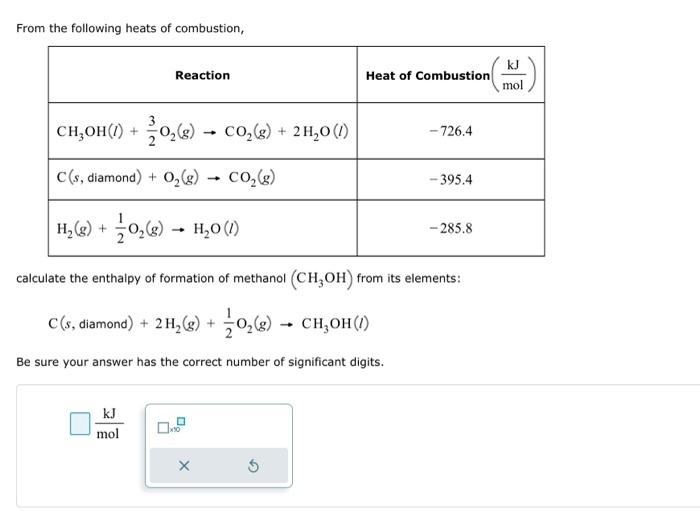
From the following heats of combustion, calculate the enthalpy of formation of methanol \( \left(\mathrm{CH}_{3} \mathrm{OH}\right) \) from its elements: \[ \mathrm{C}(\mathrm{s} \text {, diamond })+2 \mathrm{H}_{2}(\mathrm{~g})+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CH}_{3} \mathrm{OH}(\mathrm{l}) \] Be sure your answer has the correct number of significant digits.
A \( 6.62 \mathrm{~kg} \) piece of copper metal is heated from \( 19.5{ }^{\circ} \mathrm{C} \) to \( 312.3^{\circ} \mathrm{C} \). Calculate the heat absorbed (in \( \mathrm{kJ} \) ) by the metal. Be sure your answer has the correct number of significant digits. Note: Reference the Phase change properties of pure substances table for additional information.