Home /
Expert Answers /
Chemistry /
from-the-following-enthalpies-of-reaction-begin-array-ll-mathrm-h-2-mathrm-g-mathr-pa583
(Solved): From the following enthalpies of reaction: \[ \begin{array}{ll} \mathrm{H}_{2}(\mathrm{~g})+\mathr ...
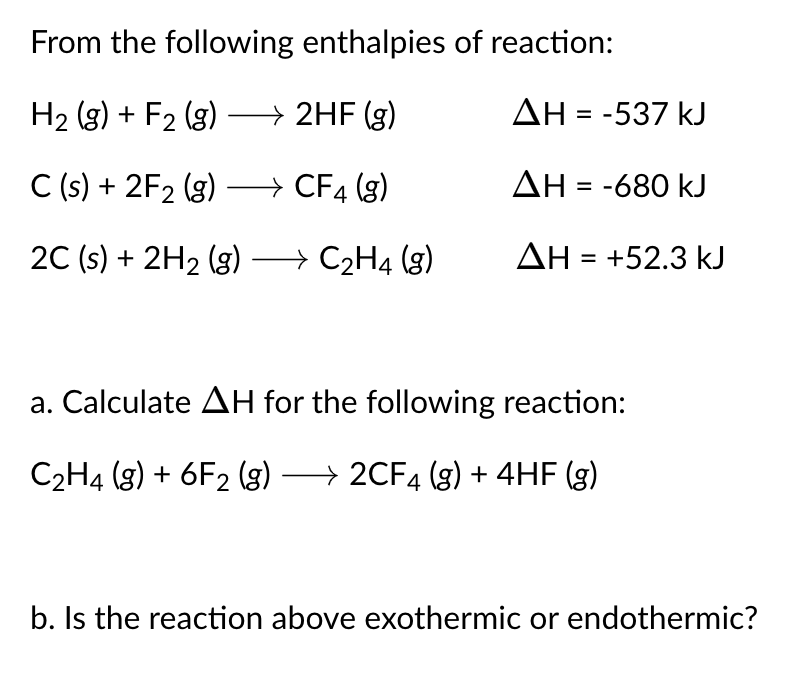
From the following enthalpies of reaction: \[ \begin{array}{ll} \mathrm{H}_{2}(\mathrm{~g})+\mathrm{F}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{HF}(\mathrm{g}) & \Delta \mathrm{H}=-537 \mathrm{~kJ} \\ \mathrm{C}(\mathrm{s})+2 \mathrm{~F}_{2}(\mathrm{~g}) \longrightarrow \mathrm{CF}_{4}(\mathrm{~g}) & \Delta \mathrm{H}=-680 \mathrm{~kJ} \\ 2 \mathrm{C}(\mathrm{s})+2 \mathrm{H}_{2}(\mathrm{~g}) \longrightarrow \mathrm{C}_{2} \mathrm{H}_{4}(\mathrm{~g}) & \Delta \mathrm{H}=+52.3 \mathrm{~kJ} \end{array} \] a. Calculate \( \Delta H \) for the following reaction: \[ \mathrm{C}_{2} \mathrm{H}_{4}(\mathrm{~g})+6 \mathrm{~F}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{CF}_{4}(\mathrm{~g})+4 \mathrm{HF}(\mathrm{g}) \] b. Is the reaction above exothermic or endothermic?