Home /
Expert Answers /
Chemistry /
for-the-reaction-ch3br-oh-ch3oh-br-the-concentration-of-ch3br-was-tracked-over-time-in-minut-pa447
(Solved): For the reaction CH3Br + OH CH3OH + Br the concentration of CH3Br was tracked over time in minut ...
For the reaction CH3Br + OH ? CH3OH + Br the concentration of CH3Br was tracked over time in minutes. The attached graph displays the concentration as CH3Br is consumed. From this, estimate the rate of the reaction (+/- 2x10-4) in M(CH3Br)/min between 0.000 min and 5.00 min. 0.025 0.02 0.015 f 0.01 0.005 0 0 5. [CH3Br] vs time y=-0.0005x + 0.0171 10 15 20 25 ****** 30 35 M(CH3Br)/minute. Your answer should be negative, because CH3Br is being consumed.
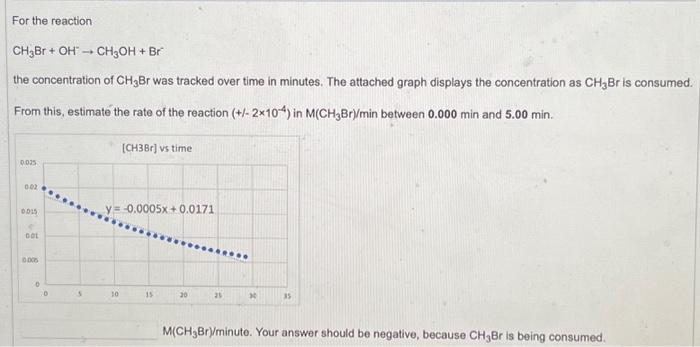
For the reaction the concentration of was tracked over time in minutes. The attached graph displays the concentration as is consumed. From this, estimate the rate of the reaction in between and .
Expert Answer
The rate of a chemical reaction is the rate of increase or decrease in the concentration. It is the ...