Home /
Expert Answers /
Chemistry /
for-the-reaction-2-mathrm-c-2-mathrm-h-6-g-7-mathrm-o-2-g-rightarrow-4-mathrm-pa494
(Solved): For the reaction: \[ 2 \mathrm{C}_{2} \mathrm{H}_{6}(g)+7 \mathrm{O}_{2}(g) \rightarrow 4 \mathrm{ ...
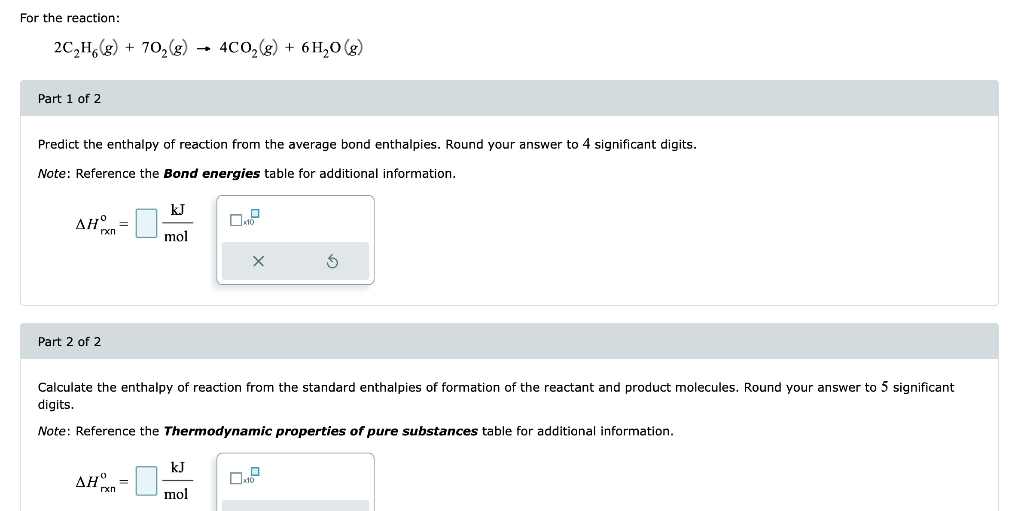
For the reaction: \[ 2 \mathrm{C}_{2} \mathrm{H}_{6}(g)+7 \mathrm{O}_{2}(g) \rightarrow 4 \mathrm{CO}_{2}(g)+6 \mathrm{H}_{2} \mathrm{O}(g) \] Part 1 of 2 Predict the enthalpy of reaction from the average bond enthalpies. Round your answer to 4 significant digits. Note: Reference the Bond energies table for additional information. \[ \Delta H_{\mathrm{rxn}}^{\mathrm{o}}=\frac{\mathrm{kJ}}{\mathrm{mol}} \] Part 2 of 2 Calculate the enthalpy of reaction from the standard enthalpies of formation of the reactant and product molecules. Round your answer to 5 significant digits. Note: Reference the Thermodynamic properties of pure substances table for additional information. \[ \Delta H_{\mathrm{rxn}}^{\mathrm{o}}=\quad \frac{\mathrm{kJ}}{\mathrm{mol}} \]