Home /
Expert Answers /
Chemistry /
for-the-following-reaction-the-products-are-favored-at-equilibrium-classify-each-of-the-reactants-pa647
(Solved): For the following reaction, the products are favored at equilibrium. Classify each of the reactants ...
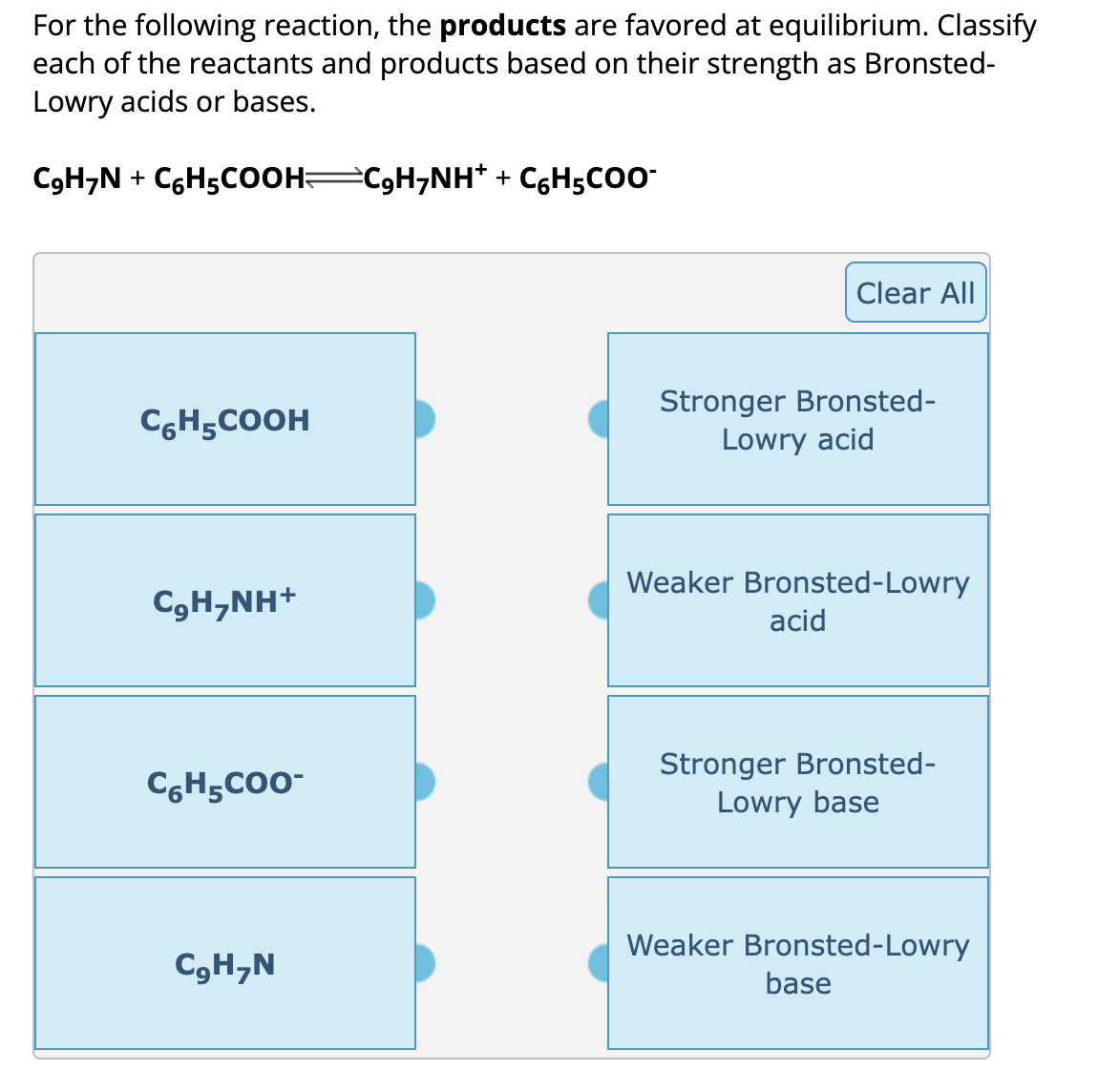
For the following reaction, the products are favored at equilibrium. Classify each of the reactants and products based on their strength as BronstedLowry acids or bases.
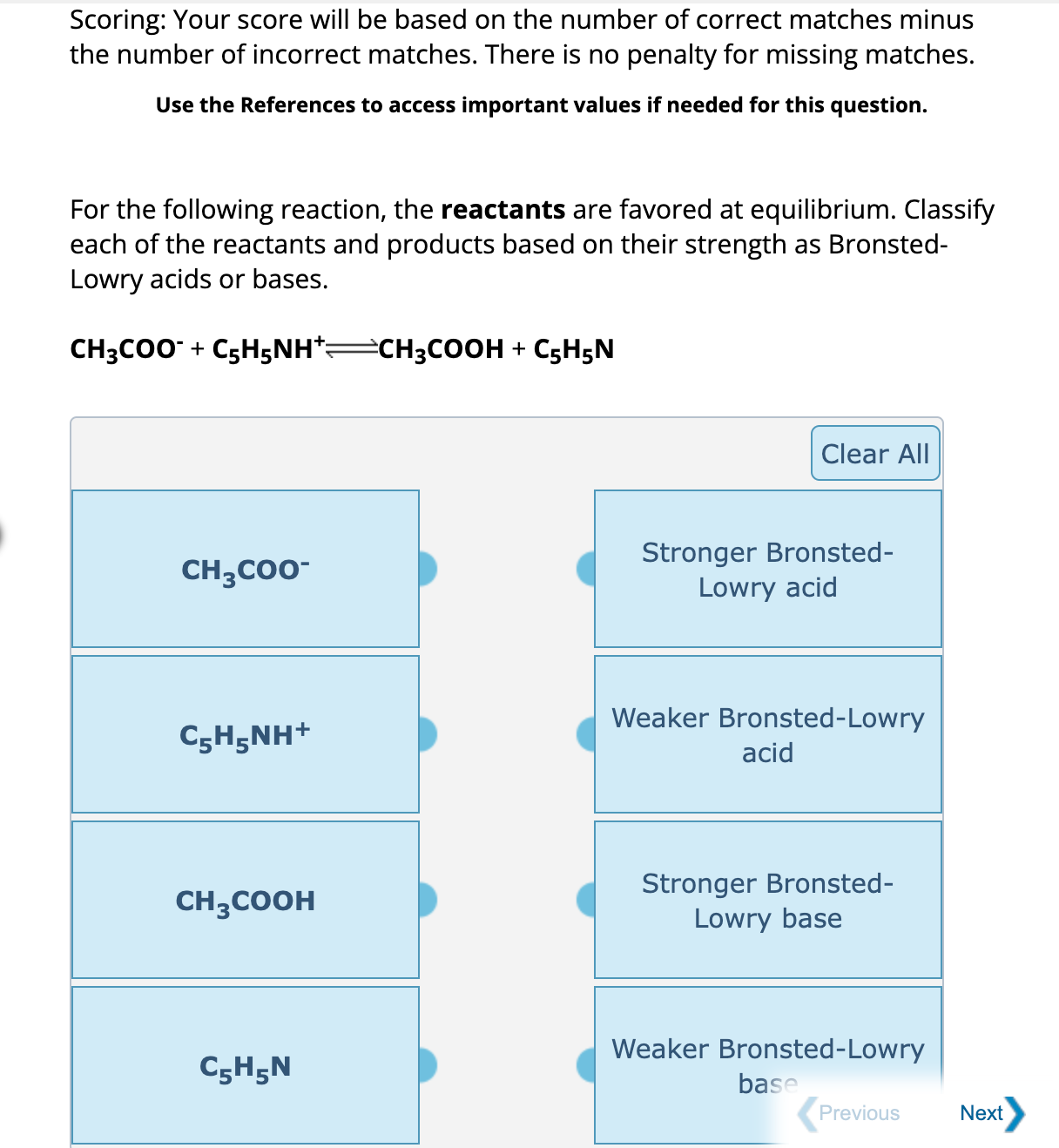
Scoring: Your score will be based on the number of correct matches minus the number of incorrect matches. There is no penalty for missing matches. Use the References to access important values if needed for this question. For the following reaction, the reactants are favored at equilibrium. Classify each of the reactants and products based on their strength as BronstedLowry acids or bases.