Home /
Expert Answers /
Chemistry /
for-the-following-reaction-h3po4-3-koh-k3po4-3-ho-1-what-is-the-equivalent-mass-of-h3po4-pa568
(Solved): For the following reaction: H3PO4 + 3 KOH-K3PO4 + 3 HO (1) What is the equivalent mass of H3PO4? ...
For the following reaction: H3PO4 + 3 KOH-K3PO4 + 3 H?O (1) What is the equivalent mass of H3PO4? (2) Calculate the number of equivalents in 35.1 grams of H3PO4. Submit Answer g/equiv Retry Entire Group 9 more group attempts remaining equiv
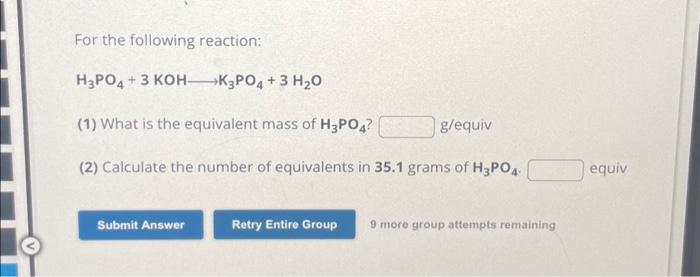
For the following reaction: (1) What is the equivalent mass of ? g/equiv (2) Calculate the number of equivalents in of . equiv 9 more group attempts remaining
Expert Answer
Step 1: GivenMass of H3PO4 = 35.1 gram For an acid basicity is defined as the number of H+ ions given by an acid.