Home /
Expert Answers /
Chemistry /
for-the-following-reaction-5-84-grams-of-silicon-tetrafluoride-are-mixed-with-excess-water-the-r-pa944
(Solved): For the following reaction, 5.84 grams of silicon tetrafluoride are mixed with excess water. The r ...
For the following reaction, 5.84 grams of silicon tetrafluoride are mixed with excess water. The reaction yields 3.04 grams of hydrofluoric acid. silicon tetrafluoride (s) + water (1) hydrofluoric acid (aq) + silicon dioxide (s) What is the theoretical yield of hydrofluoric acid ? grams What is the percent yield of hydrofluoric acid ? Visited %
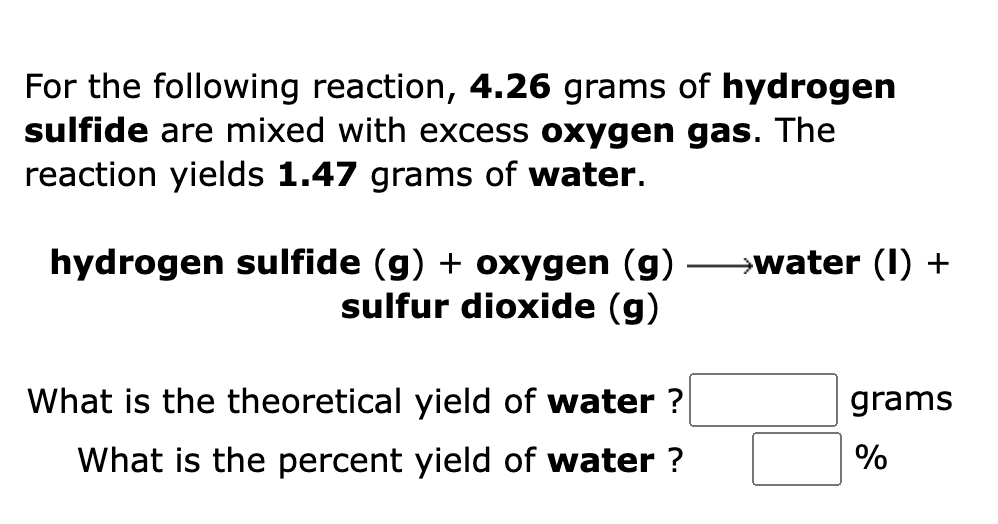
For the following reaction, 4.26 grams of hydrogen sulfide are mixed with excess oxygen gas. The reaction yields 1.47 grams of water. hydrogen sulfide (g) + oxygen (g) ????water (1) + sulfur dioxide (g) What is the theoretical yield of water? grams What is the percent yield of water? %