Home /
Expert Answers /
Chemistry /
for-the-decomposition-of-hydrogen-peroxide-in-dilute-sodium-hydroxide-at-20-c-2-h2oz-aq-2-h2o-pa369
(Solved): For the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 C 2 H2Oz(aq)2 H2O ...
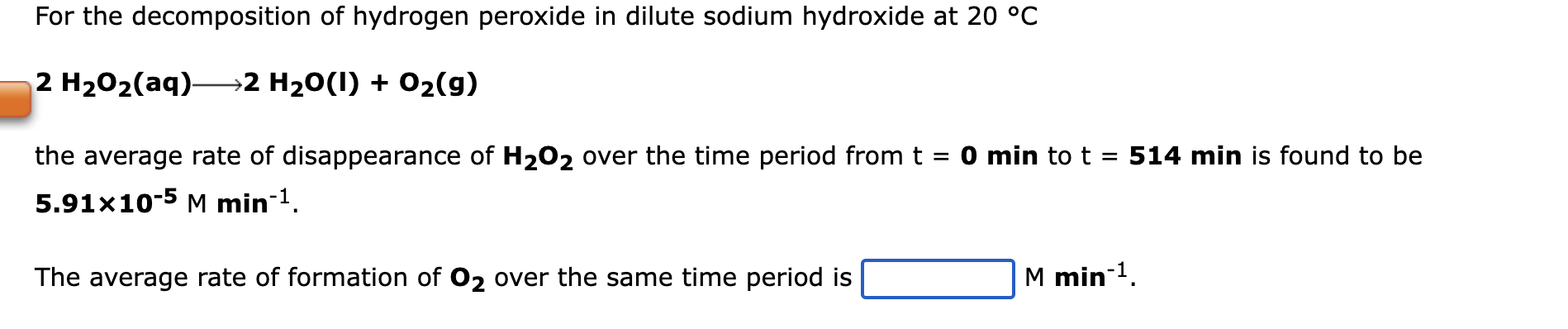
For the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 °C 2 H2Oz(aq)—2 H2O(I) + Oz(g) the average rate of disappearance of H?O2 over the time period from t = 0 min to t = 514 min is found to be 5.91x10-5 M min-¹. The average rate of formation of O2 over the same time period is M min-¹.
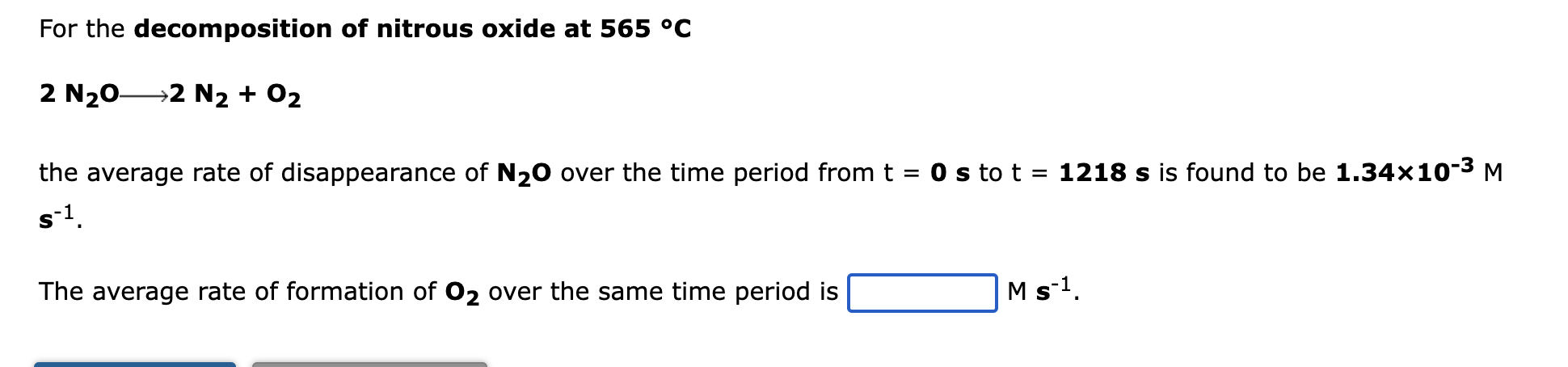
For the decomposition of nitrous oxide at 565 °C 2 N?0- ?2 N2 + O2 the average rate of disappearance of N?O over the time period from t = 0 s to t = 1218 s is found to be 1.34×10-³ M s-1. The average rate of formation of O2 over the same time period is M s-¹.
Expert Answer
Given that rate of disappearance of hydrogen peroxide is 5.91 × 10-5